Mycobacterium tuberculosis Inhibits RAB7 Recruitment to Selectively Modulate Autophagy Flux in Macrophages
- PMID: 26541268
- PMCID: PMC4635374
- DOI: 10.1038/srep16320
Mycobacterium tuberculosis Inhibits RAB7 Recruitment to Selectively Modulate Autophagy Flux in Macrophages
Abstract
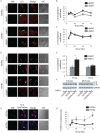
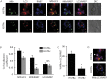
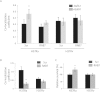
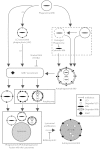
Here we report a novel regulatory mechanism for autophagy-mediated degradation of Mycobacterium tuberculosis (Mtb) and specific strategy exploited by the virulent Mtb to evade it. We show while both avirulent (H37Ra) and virulent (H37Rv) mycobacteria could readily localize to autophagosomes, their maturation into autolysosomes (flux) was significantly inhibited by the latter strain. The inhibition of autophagy flux by the virulent strain was highly selective, as it did not perturb the basal autophagy flux in the macrophages. Selective inhibition of flux of Mtb-containing autophagosomes required virulence regulators PhoP and ESAT-6. We show that the maturation of Mtb-containing autophagosomes into autolysosomes required recruitment of the late endosome marker RAB7, forming the intermediate compartment amphisomes. Virulent Mtb selectively evaded their targeting to the amphisomes. Thus we report a crosstalk between autophagy and phagosome maturation pathway and highlight the adaptability of Mtb, manifested by selective regulation of autophagy flux.
Figures

References
-
- Mostowy S. & Cossart P. Bacterial autophagy: restriction or promotion of bacterial replication? Trends Cell Biol 22, 283–291 (2012). - PubMed
-
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162 (2005). - PubMed
-
- Baxt L. A., Garza-Mayers A. C. & Goldberg M. B. Bacterial subversion of host innate immune pathways. Science 340, 697–701 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

