Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein
- PMID: 26541285
- PMCID: PMC4636065
- DOI: 10.1186/s12967-015-0705-8
Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein
Abstract
Background: Virus-like particles (VLPs) based on Newcastle disease virus (NDV) core proteins, M and NP, and containing two chimera proteins, F/F and H/G, composed of the respiratory syncytial virus (RSV) fusion protein (F) and glycoprotein (G) ectodomains fused to the transmembrane and cytoplasmic domains of the NDV F and HN proteins, respectively, stimulate durable, protective anti-RSV neutralizing antibodies in mice. Furthermore, immunization of mice with a VLP containing a F/F chimera protein with modifications previously reported to stabilize the pre-fusion form of the RSV F protein resulted in significantly improved neutralizing antibody titers over VLPs containing the wild type F protein. The goal of this study was to determine if VLPs containing the pre-fusion form of the RSV F protein stimulated protective immune responses in cotton rats, a more RSV permissive animal model than mice.
Methods: Cotton rats were immunized intramuscularly with VLPs containing stabilized pre-fusion F/F chimera protein as well as the H/G chimera protein. The anti-RSV F and RSV G antibody responses were determined by ELISA. Neutralizing antibody titers in sera of immunized animals were determined in plaque reduction assays. Protection of the animals from RSV challenge was assessed. The safety of the VLP vaccine was determined by monitoring lung pathology upon RSV challenge of immunized animals.
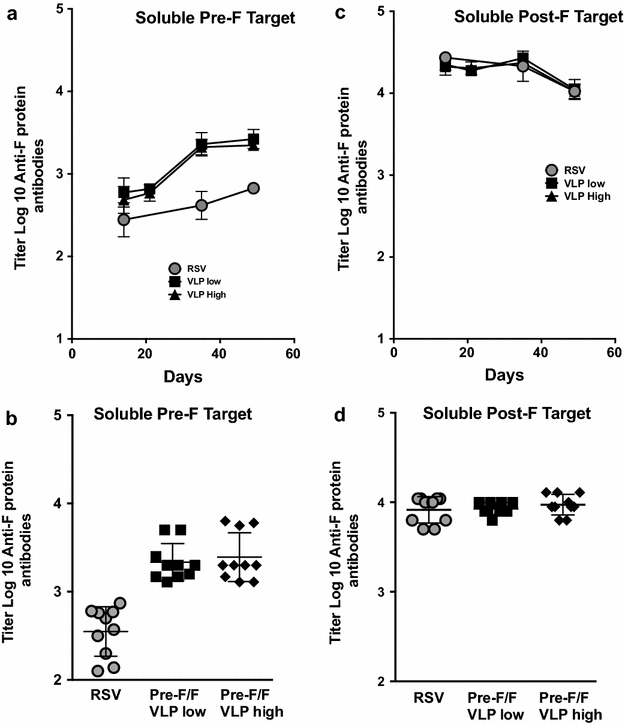
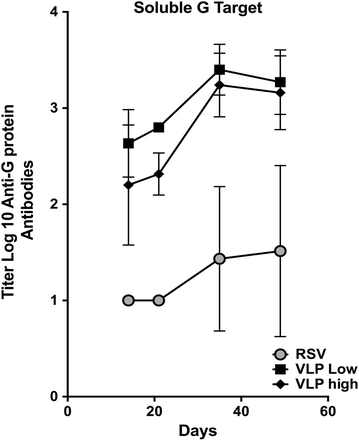
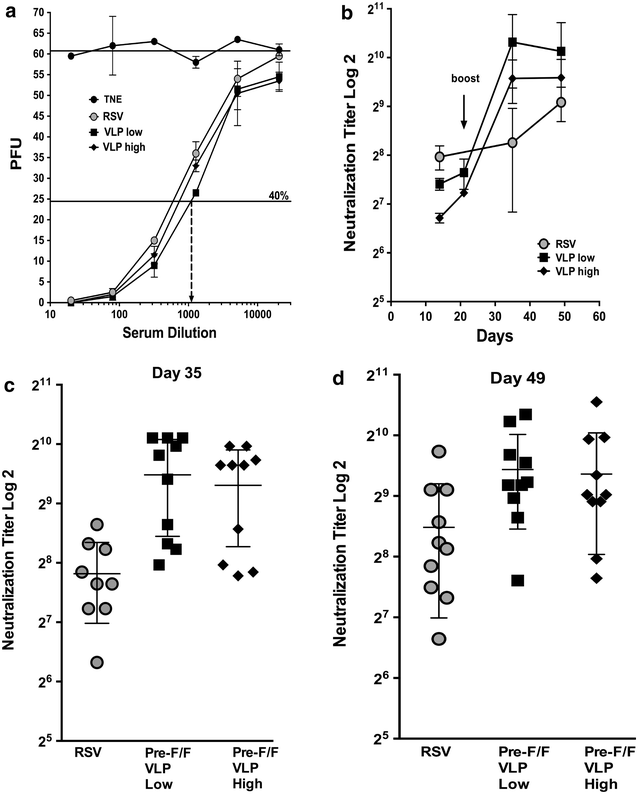
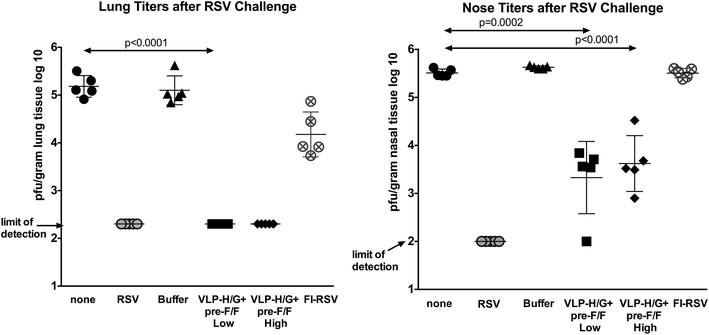
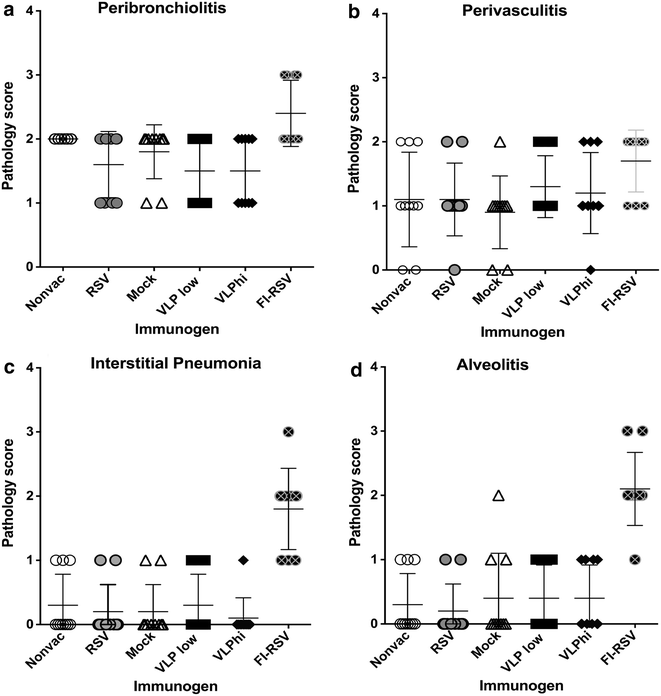
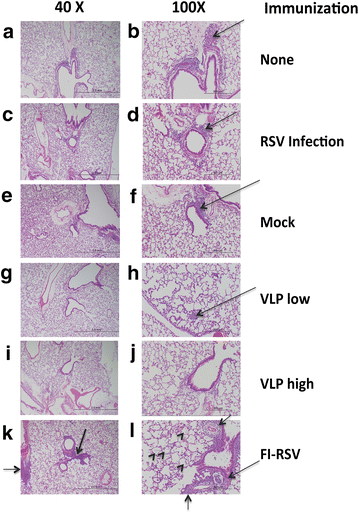
Results: The Pre-F/F VLP induced neutralizing titers that were well above minimum levels previously proposed to be required for a successful vaccine and titers significantly higher than those stimulated by RSV infection. In addition, Pre-F/F VLP immunization stimulated higher IgG titers to the soluble pre-fusion F protein than RSV infection. Cotton rats immunized with Pre-F/F VLPs were protected from RSV challenge, and, importantly, the VLP immunization did not result in enhanced respiratory disease upon RSV challenge.
Conclusions: VLPs containing the pre-fusion RSV F protein have characteristics required for a safe, effective RSV vaccine.
Figures
Similar articles
-
Alternative Virus-Like Particle-Associated Prefusion F Proteins as Maternal Vaccines for Respiratory Syncytial Virus.J Virol. 2019 Nov 13;93(23):e00914-19. doi: 10.1128/JVI.00914-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511382 Free PMC article.
-
Murine immune responses to virus-like particle-associated pre- and postfusion forms of the respiratory syncytial virus F protein.J Virol. 2015 Jul;89(13):6835-47. doi: 10.1128/JVI.00384-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903340 Free PMC article.
-
Modification of the respiratory syncytial virus f protein in virus-like particles impacts generation of B cell memory.J Virol. 2014 Sep 1;88(17):10165-76. doi: 10.1128/JVI.01250-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965456 Free PMC article.
-
RI-002, an intravenous immunoglobulin containing high titer neutralizing antibody to RSV and other respiratory viruses for use in primary immunodeficiency disease and other immune compromised populations.Expert Rev Clin Immunol. 2017 Dec;13(12):1107-1119. doi: 10.1080/1744666X.2017.1389647. Epub 2017 Oct 16. Expert Rev Clin Immunol. 2017. PMID: 29035131 Free PMC article. Review.
-
Virus-like particles: a versatile and effective vaccine platform.Expert Rev Vaccines. 2025 Dec;24(1):444-456. doi: 10.1080/14760584.2025.2508517. Epub 2025 May 22. Expert Rev Vaccines. 2025. PMID: 40387310 Review.
Cited by
-
Soluble F proteins exacerbate pulmonary histopathology after vaccination upon respiratory syncytial virus challenge but not when presented on virus-like particles.Hum Vaccin Immunother. 2017 Nov 2;13(11):2594-2605. doi: 10.1080/21645515.2017.1362514. Hum Vaccin Immunother. 2017. PMID: 28854003 Free PMC article.
-
New Insights Contributing to the Development of Effective Vaccines and Therapies to Reduce the Pathology Caused by hRSV.Int J Mol Sci. 2017 Aug 11;18(8):1753. doi: 10.3390/ijms18081753. Int J Mol Sci. 2017. PMID: 28800119 Free PMC article. Review.
-
Synthetic Biodegradable Microparticle and Nanoparticle Vaccines against the Respiratory Syncytial Virus.Vaccines (Basel). 2016 Dec 2;4(4):45. doi: 10.3390/vaccines4040045. Vaccines (Basel). 2016. PMID: 27918420 Free PMC article. Review.
-
Antigen persistence and TLR stimulation contribute to induction of a durable HIV-1-specific neutralizing antibody response.Nat Commun. 2025 Jun 3;16(1):5162. doi: 10.1038/s41467-025-60481-2. Nat Commun. 2025. PMID: 40461490 Free PMC article.
-
Novel Respiratory Syncytial Virus-Like Particle Vaccine Composed of the Postfusion and Prefusion Conformations of the F Glycoprotein.Clin Vaccine Immunol. 2016 Jun 6;23(6):451-9. doi: 10.1128/CVI.00720-15. Print 2016 Jun. Clin Vaccine Immunol. 2016. PMID: 27030590 Free PMC article.
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

