Mechanisms underlying the activity-dependent regulation of locomotor network performance by the Na+ pump
- PMID: 26541477
- PMCID: PMC4635428
- DOI: 10.1038/srep16188
Mechanisms underlying the activity-dependent regulation of locomotor network performance by the Na+ pump
Erratum in
-
Corrigendum: Mechanisms underlying the activity-dependent regulation of locomotor network performance by the Na+ pump.Sci Rep. 2017 Dec 22;7:46909. doi: 10.1038/srep46909. Sci Rep. 2017. PMID: 29269940 Free PMC article.
Abstract
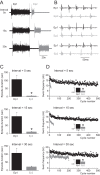
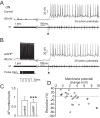
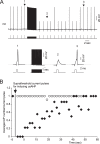
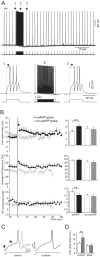
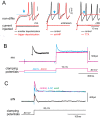
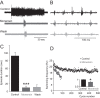
Activity-dependent modification of neural network output usually results from changes in neurotransmitter release and/or membrane conductance. In Xenopus frog tadpoles, spinal locomotor network output is adapted by an ultraslow afterhyperpolarization (usAHP) mediated by an increase in Na(+) pump current. Here we systematically explore how the interval between two swimming episodes affects the second episode, which is shorter and slower than the first episode. We find the firing reliability of spinal rhythmic neurons to be lower in the second episode, except for excitatory descending interneurons (dINs). The sodium/proton antiporter, monensin, which potentiates Na(+) pump function, induced similar effects to short inter-swim intervals. A usAHP induced by supra-threshold pulses reduced neuronal firing reliability during swimming. It also increased the threshold current for spiking and introduced a delay to the first spike in a train, without reducing subsequent firing frequency. This delay was abolished by ouabain or zero K(+) saline, which eliminate the usAHP. We present evidence for an A-type K(+) current in spinal CPG neurons which is inactivated by depolarization and de-inactivated by hyperpolarization, and accounts for the prolonged delay. We conclude that the usAHP attenuates neuronal responses to excitatory network inputs by both membrane hyperpolarization and enhanced de-inactivation of an A-current.
Figures


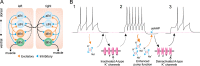
References
-
- Parker D., Hill R. & Grillner S. Electrogenic pump and a Ca2+- dependent K+ conductance contribute to a posttetanic hyperpolarization in lamprey sensory neurons. J. Neurophysiol. 76, 540–553 (1996). - PubMed
-
- Ballerini L., Bracci E. & Nistri A. Pharmacological block of the electrogenic sodium pump disrupts rhythmic bursting induced by strychnine and bicuculline in the neonatal rat spinal cord. J. Neurophysiol. 77, 17–23 (1997). - PubMed
-
- Hess D., Nanou E. & El Manira A. Characterization of Na+-activated K+ currents in larval lamprey spinal cord neurons. J. Neurophysiol. 97, 3484–3493 (2007). - PubMed
-
- Scuri R. et al. Inhibition of Na+/K+ ATPase potentiates synaptic transmission in tactile sensory neurons of the leech. Eur. J Neurosci. 25, 159–167 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

