Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation
- PMID: 26541810
- PMCID: PMC4635351
- DOI: 10.1038/srep15985
Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation
Abstract
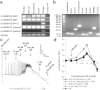
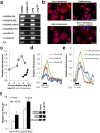
Bitter taste receptors (TAS2Rs) are present in extra-oral tissues, including gut endocrine cells. This study explored the presence and mechanism of action of TAS2R agonists on gut smooth muscle in vitro and investigated functional effects of intra-gastric administration of TAS2R agonists on gastric motility and satiation. TAS2Rs and taste signalling elements were expressed in smooth muscle tissue along the mouse gut and in human gastric smooth muscle cells (hGSMC). Bitter tastants induced concentration and region-dependent contractility changes in mouse intestinal muscle strips. Contractions induced by denatonium benzoate (DB) in gastric fundus were mediated via increases in intracellular Ca(2+) release and extracellular Ca(2+)-influx, partially masked by a hyperpolarizing K(+)-efflux. Intra-gastric administration of DB in mice induced a TAS2R-dependent delay in gastric emptying. In hGSMC, bitter compounds evoked Ca(2+)-rises and increased ERK-phosphorylation. Healthy volunteers showed an impaired fundic relaxation in response to nutrient infusion and a decreased nutrient volume tolerance and increased satiation during an oral nutrient challenge test after intra-gastric DB administration. These findings suggest a potential role for intestinal TAS2Rs as therapeutic targets to alter gastrointestinal motility and hence to interfere with hunger signalling.
Figures




References
-
- Chandrashekar J., Hoon M. A., Ryba N. J. & Zuker C. S. The receptors and cells for mammalian taste. Nature 444, 288–294 (2006). - PubMed
-
- Scott T. R. & Giza B. K. Issues of gustatory neural coding: where they stand today. Physiol. Behav. 69, 65–76 (2000). - PubMed
-
- Janssen S. & Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 24, 92–100 (2013). - PubMed
-
- Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut 63, 179–190 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

