En1 directs superior olivary complex neuron positioning, survival, and expression of FoxP1
- PMID: 26542008
- PMCID: PMC4688081
- DOI: 10.1016/j.ydbio.2015.10.008
En1 directs superior olivary complex neuron positioning, survival, and expression of FoxP1
Abstract
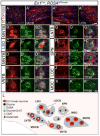
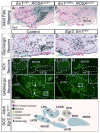
Little is known about the genetic pathways and transcription factors that control development and maturation of central auditory neurons. En1, a gene expressed by a subset of developing and mature superior olivary complex (SOC) cells, encodes a homeodomain transcription factor important for neuronal development in the midbrain, cerebellum, hindbrain and spinal cord. Using genetic fate-mapping techniques, we show that all En1-lineal cells in the SOC are neurons and that these neurons are glycinergic, cholinergic and GABAergic in neurotransmitter phenotype. En1 deletion does not interfere with specification or neural fate of these cells, but does cause aberrant positioning and subsequent death of all En1-lineal SOC neurons by early postnatal ages. En1-null cells also fail to express the transcription factor FoxP1, suggesting that FoxP1 lies downstream of En1. Our data define important roles for En1 in the development and maturation of a diverse group of brainstem auditory neurons.
Keywords: Auditory; Brainstem; Deafness; Hearing; Nucleogenesis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Atlas, A.B. n.d. http://mouse.brain-map.org.
-
- Bledsoe SC, Snead CR, Helfert RH, Prasad V, Wenthold RJ, Altschuler RA. Immunocytochemical and lesion studies support the hypothesis that the projection from the medial nucleus of the trapezoid body to the lateral superior olive is glycinergic. Brain Research. 1990;517:189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

