Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development
- PMID: 26542011
- PMCID: PMC4688075
- DOI: 10.1016/j.ydbio.2015.10.022
Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development
Abstract
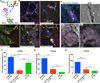
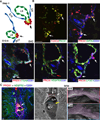
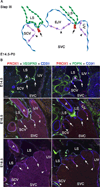
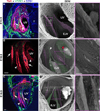
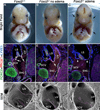
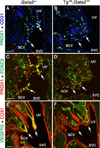
Lymph is returned to the blood circulation exclusively via four lymphovenous valves (LVVs). Despite their vital importance, the architecture and development of LVVs is poorly understood. We analyzed the formation of LVVs at the molecular and ultrastructural levels during mouse embryogenesis and identified three critical steps. First, LVV-forming endothelial cells (LVV-ECs) differentiate from PROX1(+) progenitors and delaminate from the luminal side of the veins. Second, LVV-ECs aggregate, align perpendicular to the direction of lymph flow and establish lympho-venous connections. Finally, LVVs mature with the recruitment of mural cells. LVV morphogenesis is disrupted in four different mouse models of primary lymphedema and the severity of LVV defects correlate with that of lymphedema. In summary, we have provided the first and the most comprehensive analysis of LVV development. Furthermore, our work suggests that aberrant LVVs contribute to lymphedema.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nature reviews Molecular cell biology. 2007;8:464–478. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental cell. 2011;21:193–215. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

