P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2
- PMID: 26542019
- PMCID: PMC4696497
- DOI: 10.1242/jcs.175968
P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2
Abstract
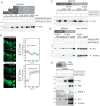
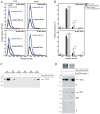
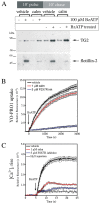
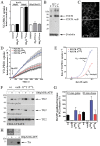
Transglutaminases (denoted TG or TGM) are externalized from cells via an unknown unconventional secretory pathway. Here, we show for the first time that purinergic signaling regulates active secretion of TG2 (also known as TGM2), an enzyme with a pivotal role in stabilizing extracellular matrices and modulating cell-matrix interactions in tissue repair. Extracellular ATP promotes TG2 secretion by macrophages, and this can be blocked by a selective antagonist against the purinergic receptor P2X7 (P2X7R, also known as P2RX7). Introduction of functional P2X7R into HEK293 cells is sufficient to confer rapid, regulated TG2 export. By employing pharmacological agents, TG2 release could be separated from P2X7R-mediated microvesicle shedding. Neither Ca(2+) signaling alone nor membrane depolarization triggered TG2 secretion, which occurred only upon receptor membrane pore formation and without pannexin channel involvement. A gain-of-function mutation in P2X7R associated with autoimmune disease caused enhanced TG2 externalization from cells, and this correlated with increased pore activity. These results provide a mechanistic explanation for a link between active TG2 secretion and inflammatory responses, and aberrant enhanced TG2 activity in certain autoimmune conditions.
Keywords: Extracellular matrix stabilization; Innate immunity; P2X7 receptor; Purinergic signaling; Transglutaminase; Unconventional protein secretion.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




Similar articles
-
P2X7 receptor-mediated TG2 externalization: a link to inflammatory arthritis?Amino Acids. 2017 Mar;49(3):453-460. doi: 10.1007/s00726-016-2319-8. Epub 2016 Aug 25. Amino Acids. 2017. PMID: 27562793 Free PMC article. Review.
-
External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification.J Biol Chem. 2005 Apr 15;280(15):15004-12. doi: 10.1074/jbc.M500962200. Epub 2005 Feb 3. J Biol Chem. 2005. PMID: 15691824
-
A possible role of transglutaminase 2 in the nucleus of INS-1E and of cells of human pancreatic islets.J Proteomics. 2014 Jan 16;96(100):314-27. doi: 10.1016/j.jprot.2013.11.011. Epub 2013 Nov 27. J Proteomics. 2014. PMID: 24291354 Free PMC article.
-
Tissue transglutaminase (TG2) protects cardiomyocytes against ischemia/reperfusion injury by regulating ATP synthesis.Cell Death Differ. 2006 Oct;13(10):1827-9. doi: 10.1038/sj.cdd.4401889. Epub 2006 Mar 10. Cell Death Differ. 2006. PMID: 16528383 No abstract available.
-
Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family.ScientificWorldJournal. 2014 Mar 23;2014:714561. doi: 10.1155/2014/714561. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24778599 Free PMC article. Review.
Cited by
-
Osteoclast Multinucleation: Review of Current Literature.Int J Mol Sci. 2020 Aug 8;21(16):5685. doi: 10.3390/ijms21165685. Int J Mol Sci. 2020. PMID: 32784443 Free PMC article. Review.
-
The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment.Cancers (Basel). 2021 Jun 3;13(11):2788. doi: 10.3390/cancers13112788. Cancers (Basel). 2021. PMID: 34205140 Free PMC article. Review.
-
Central Role of P2Y6 UDP Receptor in Arteriolar Myogenic Tone.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1598-606. doi: 10.1161/ATVBAHA.116.307739. Epub 2016 Jun 2. Arterioscler Thromb Vasc Biol. 2016. PMID: 27255725 Free PMC article.
-
RhoA vesicle trafficking-mediated transglutaminase 2 membrane translocation promotes IgA1 mesangial deposition in IgA nephropathy.JCI Insight. 2023 Oct 9;8(19):e160374. doi: 10.1172/jci.insight.160374. JCI Insight. 2023. PMID: 37811653 Free PMC article.
-
Protective effect of lappaconitine on Freund's complete adjuvant-induced arthritis exerted through P2X7 receptor-mediated regulation of M1/M2 balance in rats.J Tradit Chin Med. 2025 Feb;45(1):39-48. doi: 10.19852/j.cnki.jtcm.2025.01.004. J Tradit Chin Med. 2025. PMID: 39957157 Free PMC article.
References
-
- Aeschlimann D. and Paulsson M. (1994). Transglutaminases: protein crosslinking enzymes in tissues and body fluids. Thromb. Haemost. 71, 402-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

