A direct interaction between fascin and microtubules contributes to adhesion dynamics and cell migration
- PMID: 26542021
- PMCID: PMC4696496
- DOI: 10.1242/jcs.175760
A direct interaction between fascin and microtubules contributes to adhesion dynamics and cell migration
Abstract
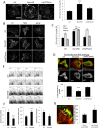
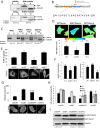
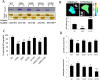
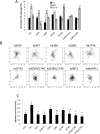
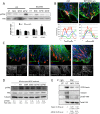
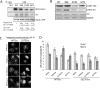
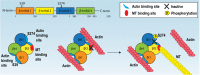
Fascin is an actin-binding and bundling protein that is highly upregulated in most epithelial cancers. Fascin promotes cell migration and adhesion dynamics in vitro and tumour cell metastasis in vivo. However, potential non-actin bundling roles for fascin remain unknown. Here, we show for the first time that fascin can directly interact with the microtubule cytoskeleton and that this does not depend upon fascin-actin bundling. Microtubule binding contributes to fascin-dependent control of focal adhesion dynamics and cell migration speed. We also show that fascin forms a complex with focal adhesion kinase (FAK, also known as PTK2) and Src, and that this signalling pathway lies downstream of fascin-microtubule association in the control of adhesion stability. These findings shed light on new non actin-dependent roles for fascin and might have implications for the design of therapies to target fascin in metastatic disease.
Keywords: Actin; Cytoskeleton; Fascin; Focal adhesion; Focal adhesion kinase; Microtubule; Migration.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
Fascin: a key regulator of cytoskeletal dynamics.Int J Biochem Cell Biol. 2010 Oct;42(10):1614-7. doi: 10.1016/j.biocel.2010.06.019. Epub 2010 Jun 30. Int J Biochem Cell Biol. 2010. PMID: 20601080 Review.
-
Interaction of fascin and protein kinase Calpha: a novel intersection in cell adhesion and motility.EMBO J. 2003 Oct 15;22(20):5390-402. doi: 10.1093/emboj/cdg521. EMBO J. 2003. PMID: 14532112 Free PMC article.
-
Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1.J Cell Sci. 1995 May;108 ( Pt 5):1977-90. doi: 10.1242/jcs.108.5.1977. J Cell Sci. 1995. PMID: 7657718
-
Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis.Mol Biol Cell. 2007 Nov;18(11):4591-602. doi: 10.1091/mbc.e07-02-0157. Epub 2007 Sep 12. Mol Biol Cell. 2007. PMID: 17855511 Free PMC article.
-
Roles of fascin in cell adhesion and motility.Curr Opin Cell Biol. 2004 Oct;16(5):590-6. doi: 10.1016/j.ceb.2004.07.009. Curr Opin Cell Biol. 2004. PMID: 15363811 Review.
Cited by
-
Strong fascin expression promotes metastasis independent of its F-actin bundling activity.Oncotarget. 2017 Nov 1;8(66):110077-110091. doi: 10.18632/oncotarget.22249. eCollection 2017 Dec 15. Oncotarget. 2017. PMID: 29299131 Free PMC article.
-
Fascin Activates β-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression.Front Oncol. 2020 Apr 21;10:440. doi: 10.3389/fonc.2020.00440. eCollection 2020. Front Oncol. 2020. PMID: 32373510 Free PMC article.
-
An optogenetic model reveals cell shape regulation through FAK and fascin.J Cell Sci. 2021 Jul 1;134(13):jcs258321. doi: 10.1242/jcs.258321. Epub 2021 Jul 8. J Cell Sci. 2021. PMID: 34114634 Free PMC article.
-
A Critical Role for the Fascin Family of Actin Bundling Proteins in Axon Development, Brain Wiring and Function.bioRxiv [Preprint]. 2025 Jun 10:2025.02.21.639554. doi: 10.1101/2025.02.21.639554. bioRxiv. 2025. Update in: Mol Cell Neurosci. 2025 Jun 17:104027. doi: 10.1016/j.mcn.2025.104027. PMID: 40027761 Free PMC article. Updated. Preprint.
-
miR-31-mediated local translation at the mitotic spindle is important for early development.Development. 2024 Sep 1;151(17):dev202619. doi: 10.1242/dev.202619. Epub 2024 Sep 5. Development. 2024. PMID: 39250531
References
-
- Byron A., Askari J. A., Humphries J. D., Jacquemet G., Koper E. J., Warwood S., Choi C. K., Stroud M. J., Chen C. S., Knight D. et al. (2015). A proteomic approach reveals integrin activation state-dependent control of microtubule cortical targeting. Nat. Commun. 6, 6135 10.1038/ncomms7135 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

