Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial
- PMID: 26542272
- PMCID: PMC4805518
- DOI: 10.1007/s00520-015-2996-y
Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial
Abstract
Purpose: Fatigue is a prevalent, distressing side effect of cancer and cancer treatment which commonly coexists with insomnia. Cognitive behavioral therapy for insomnia (CBT-I) has been shown to improve insomnia in cancer patients, but less is known about its ability to impact fatigue. This work is the analysis for a secondary aim of a four-arm randomized controlled trial (RCT) study assessing the combined and comparative effect of CBT-I and a wakefulness-promoting agent, armodafinil (A), to improve sleep and daytime functioning in cancer survivors. Herein, we examine the effect of CBT-I, with and without A, on fatigue in cancer survivors.
Patients and methods: This study was a four-arm factorial study with CBTI-I (yes/no) versus A (yes/no). It consisted of 96 cancer survivors (average age 56 years; 88 % female; 68 % breast cancer). Fatigue was assessed by the brief fatigue inventory (BFI) and the FACIT-Fatigue scale. The analysis assessed the additive effects of CBT-I and A and possible non-additive effects where the effect of CBT-I changes depending on the presence or absence of A.
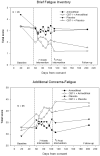
Results: Analyses adjusting for baseline differences showed that CBT-I improved fatigue as measured by two separate scales (BFI: P = 0.002, Std. error = 0.32, effect size (ES) = 0.46; FACIT-Fatigue: P < 0.001, Std. error = 1.74, ES = 0.64). Armodafinil alone did not show a statistically significant effect on fatigue levels (all Ps > 0.40) nor did the drug influence the efficacy of CBT-I. Structural equation analysis revealed that reductions in insomnia severity were directly responsible for improving cancer-related fatigue.
Conclusions: CBT-I with and without armodafinil resulted in a clinically and statistically significant reduction of subjective daytime fatigue in cancer survivors with chronic insomnia. Armodafinil did not improve cancer-related fatigue (CRF) and did not change the efficacy of CBT-I. Patients reporting CRF should be screened and, if indicated, treated for insomnia as part of a comprehensive fatigue management program.
Keywords: Armodafinil; CBT-I; Cancer; Cancer-related fatigue.
Figures

Similar articles
-
Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial.J Cancer Surviv. 2017 Jun;11(3):401-409. doi: 10.1007/s11764-017-0597-0. Epub 2017 Jan 19. J Cancer Surviv. 2017. PMID: 28105576 Free PMC article. Clinical Trial.
-
Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors.Sleep Med. 2016 Apr;20:18-24. doi: 10.1016/j.sleep.2015.12.010. Epub 2015 Dec 31. Sleep Med. 2016. PMID: 27318221 Free PMC article. Clinical Trial.
-
Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment.J Clin Oncol. 2015 Jan 10;33(2):165-71. doi: 10.1200/JCO.2014.57.6769. Epub 2014 Dec 1. J Clin Oncol. 2015. PMID: 25452447 Free PMC article. Clinical Trial.
-
Effects of cognitive-behavioral therapy for insomnia compared with controls among cancer survivors: a systematic review and meta-analysis of randomized trials.BMC Cancer. 2025 May 14;25(1):871. doi: 10.1186/s12885-025-14192-y. BMC Cancer. 2025. PMID: 40369457 Free PMC article.
-
Interventions for insomnia in cancer patients and survivors-a comprehensive systematic review and meta-analysis.JNCI Cancer Spectr. 2024 Apr 30;8(3):pkae041. doi: 10.1093/jncics/pkae041. JNCI Cancer Spectr. 2024. PMID: 38781520 Free PMC article.
Cited by
-
Hypnosis and cognitive behavioral therapy with online sessions to reduce fatigue in patients undergoing chemotherapy for a metastatic colorectal cancer: Rational and study protocol for a feasibility study.Front Psychol. 2022 Jul 27;13:953711. doi: 10.3389/fpsyg.2022.953711. eCollection 2022. Front Psychol. 2022. PMID: 35967617 Free PMC article.
-
Therapy for insomnia with chronic obstructive pulmonary disease: a randomized trial of components.J Clin Sleep Med. 2022 Dec 1;18(12):2763-2774. doi: 10.5664/jcsm.10210. J Clin Sleep Med. 2022. PMID: 35946416 Free PMC article. Clinical Trial.
-
Impact and mechanisms of cognitive behavioral therapy for insomnia on fatigue among cancer survivors: a secondary analysis of a randomized controlled trial.Sleep. 2025 Jun 13;48(6):zsaf014. doi: 10.1093/sleep/zsaf014. Sleep. 2025. PMID: 39826090 Free PMC article. Clinical Trial.
-
Cancer-Related Fatigue: Causes and Current Treatment Options.Curr Treat Options Oncol. 2020 Feb 5;21(2):17. doi: 10.1007/s11864-020-0707-5. Curr Treat Options Oncol. 2020. PMID: 32025928 Free PMC article. Review.
-
Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial.J Cancer Surviv. 2017 Jun;11(3):401-409. doi: 10.1007/s11764-017-0597-0. Epub 2017 Jan 19. J Cancer Surviv. 2017. PMID: 28105576 Free PMC article. Clinical Trial.
References
-
- Barsevick AM, Irwin MR, Hinds P, Miller A, Berger A, Jacobsen P, Ancoli-Israel S, Reeve BB, Mustian K, O'Mara A, Lai JS, Fisch M, Cella D, National Cancer Institute Clinical Trials Planning M Recommendations for high-priority research on cancer-related fatigue in children and adults. Journal of the National Cancer Institute. 2013;105(19):1432–1440. doi:10.1093/jnci/djt242. - PMC - PubMed
-
- Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, Reneman L, Schagen SB. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment--the role of fatigue. NeuroImage Clinical. 2015;7:547–554. doi:10.1016/j.nicl.2015.02.005. - PMC - PubMed
-
- Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, Springer BA, Pfalzer LA. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(10):1581–1591. doi:10.1007/s00520-010-0986-7. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

