Osteogenesis imperfecta
- PMID: 26542481
- PMCID: PMC7384887
- DOI: 10.1016/S0140-6736(15)00728-X
Osteogenesis imperfecta
Abstract
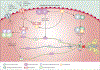
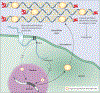
Osteogenesis imperfecta is a phenotypically and molecularly heterogeneous group of inherited connective tissue disorders that share similar skeletal abnormalities causing bone fragility and deformity. Previously, the disorder was thought to be an autosomal dominant bone dysplasia caused by defects in type I collagen, but in the past 10 years discoveries of novel (mainly recessive) causative genes have lent support to a predominantly collagen-related pathophysiology and have contributed to an improved understanding of normal bone development. Defects in proteins with very different functions, ranging from structural to enzymatic and from intracellular transport to chaperones, have been described in patients with osteogenesis imperfecta. Knowledge of the specific molecular basis of each form of the disorder will advance clinical diagnosis and potentially stimulate targeted therapeutic approaches. In this Seminar, together with diagnosis, management, and treatment, we describe the defects causing osteogenesis imperfecta and their mechanism and interrelations, and classify them into five groups on the basis of the metabolic pathway compromised, specifically those related to collagen synthesis, structure, and processing; post-translational modification; folding and cross-linking; mineralisation; and osteoblast differentiation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
We declare no competing interests.
Figures
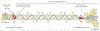

References
-
- Morello R, Bertin TK, Chen Y, et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 2006; 127: 291–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

