Epigenesis and plasticity of mouse trophoblast stem cells
- PMID: 26542801
- PMCID: PMC11108370
- DOI: 10.1007/s00018-015-2086-9
Epigenesis and plasticity of mouse trophoblast stem cells
Abstract
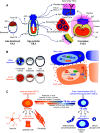
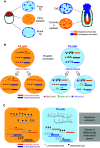
The critical role of the placenta in supporting a healthy pregnancy is mostly ensured by the extraembryonic trophoblast lineage that acts as the interface between the maternal and the foetal compartments. The diverse trophoblast cell subtypes that form the placenta originate from a single layer of stem cells that emerge from the embryo when the earliest cell fate decisions are occurring. Recent studies show that these trophoblast stem cells exhibit extensive plasticity as they are capable of differentiating down multiple pathways and are easily converted into embryonic stem cells in vitro. In this review, we discuss current knowledge of the mechanisms and control of the epigenesis of mouse trophoblast stem cells through a comparison with the corresponding mechanisms in pluripotent embryonic stem cells. To illustrate some of the more striking manifestations of the epigenetic plasticity of mouse trophoblast stem cells, we discuss them within the context of two paradigms of epigenetic regulation of gene expression: the imprinted gene expression of specific loci and the process of X-chromosome inactivation.
Keywords: Epigenetic plasticity; Mouse extraembryonic development; Multipotency; Placenta; Trophoblast stem cells.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

