Novel Pathways of Apolipoprotein A-I Metabolism in High-Density Lipoprotein of Different Sizes in Humans
- PMID: 26543096
- PMCID: PMC4690755
- DOI: 10.1161/ATVBAHA.115.306138
Novel Pathways of Apolipoprotein A-I Metabolism in High-Density Lipoprotein of Different Sizes in Humans
Abstract
Objective: A prevailing concept is that high-density lipoprotein (HDL) is secreted into the systemic circulation as a small mainly discoidal particle, which expands progressively and becomes spherical by uptake and esterification of cellular cholesterol and then contracts by cholesterol ester delivery to the liver, a process known as reverse cholesterol transport, thought to be impaired in people with low HDL cholesterol (HDLc). This metabolic framework has not been established in humans.
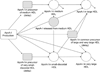
Approach and results: We studied the metabolism of apolipoprotein A-I in 4 standard HDL sizes by endogenous isotopic labeling in 6 overweight adults with low HDLc and in 6 adults with normal body weight with high plasma HDLc. Contrary to expectation, HDL was secreted into the circulation in its entire size distribution from very small to very large similarly in both groups. Very small (prebeta) HDL comprised only 8% of total apolipoprotein A-I secretion. Each HDL subfraction circulated mostly within its secreted size range for 1 to 4 days and then was cleared. Enlargement of very small and medium to large and very large HDL and generation of very small from medium HDL were minor metabolic pathways. Prebeta HDL was cleared slower, whereas medium, large, and very large HDL were cleared faster in the low HDLc group.
Conclusions: A new model is proposed from these results in which HDL is metabolized in plasma mainly within several discrete, stable sizes across the common range of HDLc concentrations.
Keywords: apolipoprotein; cholesterol; high-density lipoprotein; liver; metabolism.
© 2015 American Heart Association, Inc.
Figures
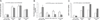




References
-
- Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. 2006;259:481–492. - PubMed
-
- AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. - PubMed
-
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

