Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation
- PMID: 26543237
- PMCID: PMC4823080
- DOI: 10.18632/oncotarget.6272
Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation
Abstract
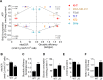
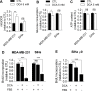
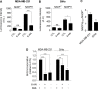
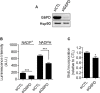
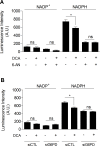
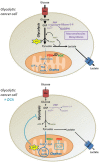
Glucose fermentation through glycolysis even in the presence of oxygen (Warburg effect) is a common feature of cancer cells increasingly considered as an enticing target in clinical development. This study aimed to analyze the link between metabolism, energy stores and proliferation rates in cancer cells. We found that cell proliferation, evaluated by DNA synthesis quantification, is correlated to glycolytic efficiency in six cancer cell lines as well as in isogenic cancer cell lines. To further investigate the link between glycolysis and proliferation, a pharmacological inhibitor of the pentose phosphate pathway (PPP) was used. We demonstrated that reduction of PPP activity decreases cancer cells proliferation, with a profound effect in Warburg-phenotype cancer cells. The crucial role of the PPP in sustaining cancer cells proliferation was confirmed using siRNAs against glucose-6-phosphate dehydrogenase, the first and rate-limiting enzyme of the PPP. In addition, we found that dichloroacetate (DCA), a new clinically tested compound, induced a switch of glycolytic cancer cells to a more oxidative phenotype and decreased proliferation. By demonstrating that DCA decreased the activity of the PPP, we provide a new mechanism by which DCA controls cancer cells proliferation.
Keywords: bioenergetics; dichloroacetate; glycolysis; pentose phosphate pathway; proliferation.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
-
- Moreno-Sanchez R, Marin-Hernandez A, Saavedra E, Pardo JP, Ralph SJ, Rodriguez-Enriquez S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int J Biochem Cell Biol. 2014;50:10–23. - PubMed
-
- Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor's dilemma? Biochim Biophys Acta. 2011;1807:552–561. - PubMed
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. - PubMed
-
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

