Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: a multicenter, double-blind, double-dummy, randomized, controlled, Phase III, non-inferiority trial
- PMID: 26543359
- PMCID: PMC4622522
- DOI: 10.2147/COPD.S90948
Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: a multicenter, double-blind, double-dummy, randomized, controlled, Phase III, non-inferiority trial
Abstract
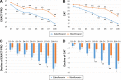
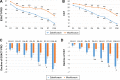
A new quinolone, zabofloxacin, has now been developed; hence, a non-inferiority trial is needed to compare this new compound with another widely used quinolone to examine its efficacy and safety for the treatment of chronic obstructive pulmonary disease (COPD) exacerbations. This was a prospective, multicenter, double-blind, double-dummy, randomized, controlled, parallel-group, Phase III, non-inferiority clinical trial designed to compare oral zabofloxacin (367 mg once daily for 5 days) with moxifloxacin (400 mg once daily for 7 days) for the treatment of patients with COPD exacerbation. In all, 345 COPD patients with a moderate COPD exacerbation were enrolled in the study via the outpatient clinics at 31 university hospitals. Clinical per protocol analysis revealed that the clinical cure rate for zabofloxacin was 86.7% and that for moxifloxacin was 86.3% (the rate difference, 0.4%; 95% confidence interval, -7.7%-8.6%). Intention-to-treat analysis revealed clinical cure rates of 77.1% and 77.3% (difference, -0.2%; 95% confidence interval, -9.0%-8.8%), respectively. These results confirm that zabofloxacin is not inferior to moxifloxacin. The favorable microbiological response rate for zabofloxacin was 67.4% and that for moxifloxacin was 79.5% (P=0.22). Patients in the zabofloxacin group showed better patient-oriented outcomes, as measured by EXAcerbations of Chronic Pulmonary Disease Tool-Patient-Reported Outcome and the COPD assessment test scores, than patients in the moxifloxacin group. Adverse drug reactions related to zabofloxacin occurred in 9.7% of cases and those related to moxifloxacin occurred in 9.6% of cases (P=0.97). The dropout rate due to adverse events was 0% (0/175) in the zabofloxacin group and 1.8% (3/167) in the moxifloxacin group (P=0.12). Oral zabofloxacin (367 mg once daily for 5 days) was not inferior to oral moxifloxacin (400 mg once daily for 7 days) for the treatment of patients with COPD exacerbation.
Keywords: CAT; EXACT-PRO; chronic obstructive pulmonary disease; exacerbation; quinolone; zabofloxacin.
Figures

Similar articles
-
Clinical Role of the Chronic Obstructive Pulmonary Disease Assessment Test in Prediction of the Response to Treatment for Exacerbations.J Korean Med Sci. 2020 Jan 13;35(2):e10. doi: 10.3346/jkms.2020.35.e10. J Korean Med Sci. 2020. PMID: 31920016 Free PMC article.
-
Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL).Lancet Infect Dis. 2016 Apr;16(4):421-30. doi: 10.1016/S1473-3099(16)00017-7. Epub 2016 Feb 5. Lancet Infect Dis. 2016. PMID: 26852726 Clinical Trial.
-
Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial.Respir Res. 2010 Jan 28;11(1):10. doi: 10.1186/1465-9921-11-10. Respir Res. 2010. PMID: 20109213 Free PMC article. Clinical Trial.
-
Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials.Lancet Respir Med. 2013 May;1(3):210-23. doi: 10.1016/S2213-2600(13)70040-7. Epub 2013 Apr 12. Lancet Respir Med. 2013. PMID: 24429127 Clinical Trial.
-
Moxifloxacin-Induced Visual Hallucinations: A Case Report and Review of the Literature.J Pharm Pract. 2017 Jun;30(3):375-377. doi: 10.1177/0897190016637987. Epub 2016 Mar 21. J Pharm Pract. 2017. PMID: 27000139 Review.
Cited by
-
Clinical Role of the Chronic Obstructive Pulmonary Disease Assessment Test in Prediction of the Response to Treatment for Exacerbations.J Korean Med Sci. 2020 Jan 13;35(2):e10. doi: 10.3346/jkms.2020.35.e10. J Korean Med Sci. 2020. PMID: 31920016 Free PMC article.
-
Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile?Pharmaceutics. 2023 Mar 1;15(3):804. doi: 10.3390/pharmaceutics15030804. Pharmaceutics. 2023. PMID: 36986665 Free PMC article. Review.
-
Antimicrobial Therapy in Community-Acquired Pneumonia in Children.Curr Infect Dis Rep. 2018 Sep 20;20(11):47. doi: 10.1007/s11908-018-0653-6. Curr Infect Dis Rep. 2018. PMID: 30238375 Review.
-
Emerging Treatment Options for Infections by Multidrug-Resistant Gram-Positive Microorganisms.Microorganisms. 2020 Jan 30;8(2):191. doi: 10.3390/microorganisms8020191. Microorganisms. 2020. PMID: 32019171 Free PMC article. Review.
-
Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones-Shaping the Fifth Generation.Pharmaceutics. 2021 Aug 18;13(8):1289. doi: 10.3390/pharmaceutics13081289. Pharmaceutics. 2021. PMID: 34452252 Free PMC article. Review.
References
-
- Pela R, Marchesani F, Agostinelli C, et al. Airways microbial flora in COPD patients in stable clinical conditions and during exacerbations: a bronchoscopic investigation. Monaldi Arch Chest Dis. 1998;53(3):262–267. - PubMed
-
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. - PubMed
-
- Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35(1):157–163. - PubMed
-
- Kwon AR, Min YH, Ryu JM, Choi DR, Shim MJ, Choi EC. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J Antimicrob Chemother. 2006;58(3):684–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

