Tolerogenic Dendritic Cells on Transplantation: Immunotherapy Based on Second Signal Blockage
- PMID: 26543876
- PMCID: PMC4620289
- DOI: 10.1155/2015/856707
Tolerogenic Dendritic Cells on Transplantation: Immunotherapy Based on Second Signal Blockage
Abstract
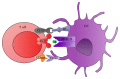
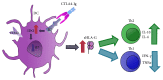
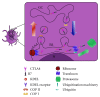
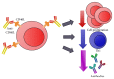
Dendritic cells (DCs), the most important professional antigen-presenting cells (APC), play crucial role in both immunity and tolerance. It is well known that DCs are able to mount immune responses against foreign antigens and simultaneously tolerate self-antigens. Since DCs can be modulated depending on the surrounding microenvironment, they can act as a bridge between innate and adaptive immunity. However, the mechanisms that support this dual role are not entirely clear. Recent studies have shown that DCs can be manipulated ex vivo in order to trigger their tolerogenic profile, what can be a tool to be used in clinical trials aiming the treatment of various diseases and the prevention of transplant rejection. In this sense, the blockage of costimulatory molecules on DC, in the attempt of inhibiting the second signal in the immunological synapse, can be considered as one of the main strategies under development. This review brings an update on current therapies using tolerogenic dendritic cells modulated with costimulatory blockers with the aim of reducing transplant rejection. However, although there are current clinical trials using tolerogenic DC to treat allograft rejection, the actual challenge is to modulate these cells in order to maintain a permanent tolerogenic profile.
Figures
Similar articles
-
Tolerogenic dendritic cells: molecular and cellular mechanisms in transplantation.J Leukoc Biol. 2014 Jan;95(1):53-69. doi: 10.1189/jlb.0613336. Epub 2013 Oct 9. J Leukoc Biol. 2014. PMID: 24108704 Review.
-
GDF15 Regulates Malat-1 Circular RNA and Inactivates NFκB Signaling Leading to Immune Tolerogenic DCs for Preventing Alloimmune Rejection in Heart Transplantation.Front Immunol. 2018 Oct 30;9:2407. doi: 10.3389/fimmu.2018.02407. eCollection 2018. Front Immunol. 2018. PMID: 30425709 Free PMC article.
-
Tolerogenic Dendritic Cells: The Pearl of Immunotherapy in Organ Transplantation.Front Immunol. 2020 Oct 6;11:552988. doi: 10.3389/fimmu.2020.552988. eCollection 2020. Front Immunol. 2020. PMID: 33123131 Free PMC article. Review.
-
[Role of innate immunity in tolerance induction].Biomed Khim. 2015 Sep-Oct;61(5):560-78. doi: 10.18097/PBMC20156105560. Biomed Khim. 2015. PMID: 26539864 Review. Russian.
-
Emerging role of innate immunity in organ transplantation part III: the quest for transplant tolerance via prevention of oxidative allograft injury and its consequences.Transplant Rev (Orlando). 2012 Apr;26(2):88-102. doi: 10.1016/j.trre.2011.07.001. Epub 2011 Oct 13. Transplant Rev (Orlando). 2012. PMID: 22000661 Review.
Cited by
-
Generation of Tolerogenic Dendritic Cells and Their Therapeutic Applications.Immune Netw. 2016 Feb;16(1):52-60. doi: 10.4110/in.2016.16.1.52. Epub 2016 Feb 25. Immune Netw. 2016. PMID: 26937232 Free PMC article. Review.
-
A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE.Sci Rep. 2017 Aug 29;7(1):9886. doi: 10.1038/s41598-017-06907-4. Sci Rep. 2017. PMID: 28851867 Free PMC article.
-
Downregulation of L-arginine metabolism in dendritic cells induces tolerance to exogenous antigen.Int J Immunopathol Pharmacol. 2017 Mar;30(1):44-57. doi: 10.1177/0394632016678873. Epub 2017 Jan 24. Int J Immunopathol Pharmacol. 2017. PMID: 27903843 Free PMC article.
-
Tolerogenic Dendritic Cells and T-Regulatory Cells at the Clinical Trials Crossroad for the Treatment of Autoimmune Disease; Emphasis on Type 1 Diabetes Therapy.Front Immunol. 2019 Feb 6;10:148. doi: 10.3389/fimmu.2019.00148. eCollection 2019. Front Immunol. 2019. PMID: 30787930 Free PMC article. Review.
-
Maternal dendritic cells influence fetal allograft response following murine in-utero hematopoietic stem cell transplantation.Stem Cell Res Ther. 2023 May 24;14(1):136. doi: 10.1186/s13287-023-03366-9. Stem Cell Res Ther. 2023. PMID: 37226255 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

