Pharmacist-Led Medication Reviews to Identify and Collaboratively Resolve Drug-Related Problems in Psychiatry - A Controlled, Clinical Trial
- PMID: 26544202
- PMCID: PMC4636233
- DOI: 10.1371/journal.pone.0142011
Pharmacist-Led Medication Reviews to Identify and Collaboratively Resolve Drug-Related Problems in Psychiatry - A Controlled, Clinical Trial
Abstract
Aim of the study: This prospective, controlled trial aimed to assess the effect of pharmacist-led medication reviews on the medication safety of psychiatric inpatients by the resolution of Drug-Related Problems (DRP). Both the therapy appropriateness measured with the Medication Appropriateness Index (MAI) and the number of unsolved DRP per patient were chosen as primary outcome measures.
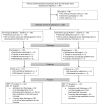
Methods: Depending on their time of admission, 269 psychiatric patients that were admitted to a psychiatric university hospital were allocated in control (09/2012-03/2013) or intervention group (05/2013-12/2013). In both groups, DRP were identified by comprehensive medication reviews by clinical pharmacists at admission, during the hospital stay, and at discharge. In the intervention group, recommendations for identified DRP were compiled by the pharmacists and discussed with the therapeutic team. In the control group, recommendations were not provided except for serious or life threatening DRP. As a primary outcome measure, the changes in therapy appropriateness from admission to discharge as well as from admission to three months after discharge (follow-up) assessed with the MAI were compared between both groups. The second primary outcome was the number of unsolved DRP per patient after completing the study protocol. The DRP type, the relevance and the potential of drugs to cause DRP were also evaluated.
Results: The intervention led to a reduced MAI score by 1.4 points per patient (95% confidence interval [CI]: 0.8-2.0) at discharge and 1.3 points (95% CI: 0.7-1.9) at follow-up compared with controls. The number of unsolved DRP in the intervention group was 1.8 (95% CI: 1.5-2.1) less than in control.
Conclusion: The pharmaceutical medication reviews with interdisciplinary discussion of identified DRP appears to be a worthy strategy to improve medication safety in psychiatry as reflected by less unsolved DRP per patient and an enhanced appropriateness of therapy. The promising results of this trial likely warrant further research that evaluates direct clinical outcomes and health-related costs.
Trial registration: Deutsches Register Klinischer Studien (DRKS), DRKS00006358.
Conflict of interest statement
Figures

References
-
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. J Am Med Assoc 1995;274:29–34. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

