EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals
- PMID: 26544531
- PMCID: PMC4702494
- DOI: 10.1210/er.2015-1010
EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals
Abstract
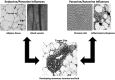
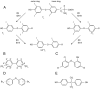
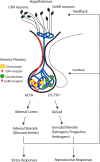
The Endocrine Society's first Scientific Statement in 2009 provided a wake-up call to the scientific community about how environmental endocrine-disrupting chemicals (EDCs) affect health and disease. Five years later, a substantially larger body of literature has solidified our understanding of plausible mechanisms underlying EDC actions and how exposures in animals and humans-especially during development-may lay the foundations for disease later in life. At this point in history, we have much stronger knowledge about how EDCs alter gene-environment interactions via physiological, cellular, molecular, and epigenetic changes, thereby producing effects in exposed individuals as well as their descendants. Causal links between exposure and manifestation of disease are substantiated by experimental animal models and are consistent with correlative epidemiological data in humans. There are several caveats because differences in how experimental animal work is conducted can lead to difficulties in drawing broad conclusions, and we must continue to be cautious about inferring causality in humans. In this second Scientific Statement, we reviewed the literature on a subset of topics for which the translational evidence is strongest: 1) obesity and diabetes; 2) female reproduction; 3) male reproduction; 4) hormone-sensitive cancers in females; 5) prostate; 6) thyroid; and 7) neurodevelopment and neuroendocrine systems. Our inclusion criteria for studies were those conducted predominantly in the past 5 years deemed to be of high quality based on appropriate negative and positive control groups or populations, adequate sample size and experimental design, and mammalian animal studies with exposure levels in a range that was relevant to humans. We also focused on studies using the developmental origins of health and disease model. No report was excluded based on a positive or negative effect of the EDC exposure. The bulk of the results across the board strengthen the evidence for endocrine health-related actions of EDCs. Based on this much more complete understanding of the endocrine principles by which EDCs act, including nonmonotonic dose-responses, low-dose effects, and developmental vulnerability, these findings can be much better translated to human health. Armed with this information, researchers, physicians, and other healthcare providers can guide regulators and policymakers as they make responsible decisions.
Figures




Comment in
-
Letter to the Editor: Response to "Second Scientific Statement on Endocrine-Disrupting Chemicals" by Gore et al.Endocr Rev. 2016 Apr;37(2):L1-2. doi: 10.1210/er.2016-1005. Endocr Rev. 2016. PMID: 27049553 No abstract available.
-
Stand firm on hormone disruptors.Nature. 2016 Nov 24;539(7630):469. doi: 10.1038/539469a. Nature. 2016. PMID: 27882994 No abstract available.
References
-
- Dodds EC, Lawson W. Synthetic oestrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

