Ethanol for preventing preterm birth in threatened preterm labor
- PMID: 26544539
- PMCID: PMC8944412
- DOI: 10.1002/14651858.CD011445.pub2
Ethanol for preventing preterm birth in threatened preterm labor
Abstract
Background: Preterm birth is the leading cause of death and disability in newborns worldwide. A wide variety of tocolytic agents have been utilized to delay birth for women in preterm labor. One of the earliest tocolytics utilized for this purpose was ethanol infusion, although this is not generally used in current practice due to safety concerns for both the mother and her baby.
Objectives: To determine the efficacy of ethanol in stopping preterm labor, preventing preterm birth, and the impact of ethanol on neonatal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2015) and reference lists of retrieved studies.
Selection criteria: We included randomized and quasi-randomized studies. Cluster-randomized trials and cross-over design trials were not eligible for inclusion. We only included studies published in abstract form if there was enough information on methods and relevant outcomes. Trials were included if they compared ethanol infusion to stop preterm labor versus placebo/control or versus other tocolytic drugs.
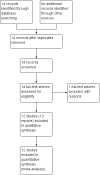
Data collection and analysis: At least two review authors independently assessed studies for inclusion and risk of bias. At least two review authors independently extracted data. Data were checked for accuracy.
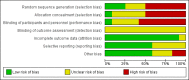
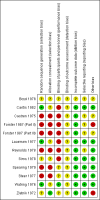
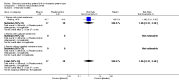
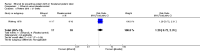
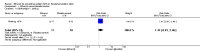
Main results: Twelve trials involving 1586 women met inclusion criteria for this review. One trial did not report on the outcomes of interest in this review.Risk of bias of included studies: The included studies generally were of low quality based on inadequate reporting of methodology. Only three trials had low risk of bias for random sequence generation and one had low risk of bias for allocation concealment and participant blinding. Most studies were either high risk of bias or uncertain in these key areas. Comparison 1: Ethanol versus placebo/control (two trials, 77 women) Compared to controls receiving pain medications and dextrose solution, ethanol did not improve any of the primary outcomes: birth < 48 hours after trial entry (one trial, 35 women; risk ratio (RR) 0.93, 95% confidence interval (CI) 0.43 to 2.00), or neonatal mortality (one trial, 35 women; RR 1.06, 95% CI 0.31 to 3.58). Serious maternal adverse events and perinatal mortality were not reported by either of the two trials in this comparison. Maternal adverse events (overall) were not reported but one trial (42 women) reported that there were no maternal adverse events that required stopping or changing drug) in either group. One trial did report delay until delivery but this outcome was reported as a median with no mention of the standard deviation (median 19 days in ethanol group versus "less than 1" day in the glucose/water group). There were no differences in any secondary outcomes reported: preterm birth < 34 weeks or < 37 weeks; serious infant outcome; fetal alcohol syndrome/fetal alcohol spectrum disorder; or small-for-gestational age. Comparison 2: Ethanol versus other tocolytic (betamimetics) (nine trials, 1438 women) Compared to betamimetics (the only tocolytic used as a comparator in these studies), ethanol was associated with no clear difference in the rate of birth < 48 hours after trial entry (two trials, 130 women; average RR 1.12, 95% CI 0.53 to 2.37, Tau² = 0.19, I² = 59%), similar rates of perinatal mortality (six trials, 698 women; RR1.20, 95% CI 0.78 to 1.84), higher rates of neonatal mortality (eight trials, 1238 women; RR 1.43, 95% CI 1.02 to 2.02), higher rates of preterm birth < 34 weeks (two trials, 599 women; RR 1.56, 95% CI 1.11 to 2.19), higher rates of neonatal respiratory distress syndrome (three trials, 823 women; RR 1.76, 95% CI 1.33 to 2.33), and higher rates of low birthweight babies < 2500 g (five trials, 834 women; RR 1.30, 95% CI 1.09 to 1.54). These outcomes are likely all related to the lower incidence of preterm birth seen with other tocolytics, which for all these comparisons were betamimetics. Serious maternal adverse events were not reported in any of the nine trial reports. However, ethanol had a trend towards a lower rate of maternal adverse events requiring stopping or changing the drug (three trials, 214 women; RR 0.25, 95% CI 0.06 to 0.97). There were no differences in other secondary outcomes of preterm birth < 37 weeks, number of days delivery was delayed, or overall maternal adverse events.Planned sensitivity analysis, excluding quasi-randomized trials did not substantially change the results of the primary outcome analyses with the exception of neonatal mortality which no longer showed a clear difference between the ethanol and other tocolytic groups (3 trials, 330 women; RR 1.49, 95% CI 0.82 to 2.72).
Authors' conclusions: This review is based on evidence from twelve studies which were mostly low quality. There is no evidence that to suggest that ethanol is an effective tocolytic compared to placebo. There is some evidence that ethanol may be better tolerated than other tocolytics (in this case betamimetics), but this result is based on few studies and small sample size and therefore should be interpreted with caution. Ethanol appears to be inferior to betamimetics for preventing preterm birth in threatened preterm labor.Ethanol is generally no longer used in current practice due to safety concerns for the mother and her baby. There is no need for new studies to evaluate the use of ethanol for preventing preterm birth in threatened preterm labour. However, it would be useful for long-term follow-up studies on the babies born to mothers from the existing studies in order to assess the risk of long-term neurodevelopmental status.
Conflict of interest statement
David M Haas: none known
Amanda Morgan: none known
Samantha Deans: none known
Frank Schubert: none known
Figures















Update of
- doi: 10.1002/14651858.CD011445
Similar articles
-
Calcium channel blockers for inhibiting preterm labour and birth.Cochrane Database Syst Rev. 2014 Jun 5;2014(6):CD002255. doi: 10.1002/14651858.CD002255.pub2. Cochrane Database Syst Rev. 2014. PMID: 24901312 Free PMC article.
-
Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour.Cochrane Database Syst Rev. 2015 Dec 14;2015(12):CD011200. doi: 10.1002/14651858.CD011200.pub2. Cochrane Database Syst Rev. 2015. PMID: 26662716 Free PMC article.
-
Oxytocin receptor antagonists for inhibiting preterm labour.Cochrane Database Syst Rev. 2014 Jun 6;2014(6):CD004452. doi: 10.1002/14651858.CD004452.pub3. Cochrane Database Syst Rev. 2014. PMID: 24903678 Free PMC article.
-
Cyclo-oxygenase (COX) inhibitors for treating preterm labour.Cochrane Database Syst Rev. 2015 Jun 5;2015(6):CD001992. doi: 10.1002/14651858.CD001992.pub3. Cochrane Database Syst Rev. 2015. PMID: 26042617 Free PMC article.
-
Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD006764. doi: 10.1002/14651858.CD006764.pub4. Cochrane Database Syst Rev. 2022. PMID: 35943347 Free PMC article.
Cited by
-
Treatment Effects in Randomized and Nonrandomized Studies of Pharmacological Interventions: A Meta-Analysis.JAMA Netw Open. 2024 Sep 3;7(9):e2436230. doi: 10.1001/jamanetworkopen.2024.36230. JAMA Netw Open. 2024. PMID: 39331390 Free PMC article.
-
A historical narrative review through the field of tocolysis in threatened preterm birth.Eur J Obstet Gynecol Reprod Biol X. 2024 Apr 29;22:100313. doi: 10.1016/j.eurox.2024.100313. eCollection 2024 Jun. Eur J Obstet Gynecol Reprod Biol X. 2024. PMID: 38736527 Free PMC article. Review.
-
Efficacy and Safety of Inhalation of Nebulized Ethanol in COVID-19 Treatment: A Randomized Clinical Trial.Cureus. 2022 Dec 5;14(12):e32218. doi: 10.7759/cureus.32218. eCollection 2022 Dec. Cureus. 2022. PMID: 36505954 Free PMC article.
-
The Intricate Interactions between Maternal Smoking and Drinking During Pregnancy and Birthweight Z-Scores of Preterm Births.J Womens Health Care Manag. 2021;2(2):10.47275/2692-0948-121. doi: 10.47275/2692-0948-121. Epub 2021 Mar 27. J Womens Health Care Manag. 2021. PMID: 34723283 Free PMC article.
-
Surrogate endpoints for neonatal outcome: A rapid review.Health Sci Rep. 2024 Aug 2;7(8):e2279. doi: 10.1002/hsr2.2279. eCollection 2024 Aug. Health Sci Rep. 2024. PMID: 39100713 Free PMC article. No abstract available.
References
References to studies included in this review
Boyd 1978 {published data only}
-
- Boyd IE, Chamberlain GV, Lewis PJ, Sims CD. Comparative trial of salbutamol and ethanol in preterm labour [abstract]. British Journal of Clinical Pharmacology 1978;5:360P‐1P. - PubMed
Caritis 1982 {published data only}
-
- Caritis SN, Carson D, Greebon D, McCormick M, Edelstone DI, Mueller‐Heubach E. A comparison of terbutaline and ethanol in the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1982;142:183‐90. - PubMed
Castren 1975 {published data only}
-
- Castren O, Gummerus M, Saarikoski S. Treatment of imminent premature labour. Acta Obstetricia et Gynecologica Scandinavica 1975;54:95‐100. - PubMed
Forster 1987 (Part II) {published data only}
-
- Forster F, During R. Comparison of the effectivity of Partusisten(Regtrademark) and ethanol tocolysis Part II: Short term tocolysis with Partusisten (Regtrademark) or ethanol. Zentralblatt fur Gynakologie 1987;109:843‐9. - PubMed
Forster 1987 (Part III) {published data only}
-
- Forster F, During R. Comparison of the effectivity of Partusisten (Regtrademark) and ethanol tocolysis Part III: Comparison of long term and short term tocolysis, using Partusisten (Regtrademark) or ethanol. Zentralblatt fur Gynakologie 1987;109:1177‐84. - PubMed
Lauersen 1977 {published data only}
-
- Fuchs F. Prevention of prematurity. American Journal of Obstetrics and Gynecology 1976;126:809‐20. - PubMed
-
- Lauersen NH, Merkatz IR, Tejani N, Wilson KH, Roberson A, Mann LI, et al. Inhibition of premature labor: A multicenter comparison of ritodrine and ethanol. American Journal of Obstetrics and Gynecology 1977;127:837‐45. - PubMed
Reynolds 1978 {published data only}
-
- Reynolds JW. A comparison of salbutamol and ethanol in the treatment of premature labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1978;18:107‐9.
Sims 1978 {published data only}
-
- Sims CD, Chamberlain GVP, Boyd IE, Lewis PJ. A comparison of salbutamol and ethanol in the treatment of preterm labour. British Journal of Obstetrics and Gynaecology 1978;85:761‐6. - PubMed
Spearing 1979 {published data only}
-
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. - PubMed
Steer 1977 {published data only}
-
- Steer CM, Petrie RH. A comparison of magnesium sulfate and alcohol for the prevention of premature labor. American Journal of Obstetrics and Gynecology 1977;129:1‐4. - PubMed
Watring 1976 {published data only}
-
- Watring WG, Benson WL, Wiebe RA, Vaughn DL. Intravenous alcohol: a single blind study in the prevention of premature delivery: a preliminary report. Journal of Reproductive Medicine 1976;16:35‐8. - PubMed
Zlatnik 1972 {published data only}
-
- Zlatnik FJ, Fuchs F. A controlled study of ethanol in threatened premature labour. American Journal of Obstetrics and Gynecology 1972;112:610‐2. - PubMed
References to studies excluded from this review
Waltman 1969 {published data only}
-
- Waltman R, Nigrin G, Bonura F, Pipat C. Ethanol in prevention of hyperbilirubinaemia in the newborn. A controlled trial. Lancet 1969;2:1265‐7. - PubMed
Additional references
ACOG 2011
-
- ACOG. American College of Obstetricians and Gynecologists. Committee on Health Care for Underserved Women. Committee opinion no. 496: At‐risk drinking and alcohol dependence: obstetric and gynecologic implications. Obstetrics and Gynecology 2011;118(2 Pt 1):383‐8. - PubMed
Bain 2013
Belinkoff 1950
-
- Belinkoff S, Hall OW Jr. Alcohol during labor. American Journal of Obstetrics and Gynecology 1950;59:429‐32.
Conde‐Agudelo 2011
Crowther 2014
Duckitt 2014
Flenady 2014a
Flenady 2014b
Fuchs 1965
-
- Fuchs F. Treatment of threatened premature labour with alcohol. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1011‐3.
Fuchs 1967
-
- Fuchs F, Fuchs AF, Poblete VF, Risk A. Effect of alcohol on threatened premature labor. American Journal of Obstetrics and Gynecology 1967;99:627‐37. - PubMed
Gladstone 2011
Haas 2012
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howson 2012
-
- Howson CP, Kinney MV, Lawn JE. March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012.
Lawn 2010
Neilson 2014
Reinebrant 2015
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
Schaefer 2007
-
- Schaefer C. Drugs During Pregnancy and Lactation. 2nd Edition. Elsevier BV, 2007.
Schulz 2010
Stan 2013
Su 2014
Vogel 2014
WHO 1977
-
- Anon. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstetricia et Gynecologica Scandinavica 1977;56(3):247‐53. - PubMed
Zeeman 1997
-
- Zeeman GG, Khan‐Dawood FS, Dawood MY. Oxytocin and its receptor in pregnancy and parturition: current concepts and clinical implications. Obstetrics and Gynecology 1997;89(5 Pt 2):873‐83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

