Characterization of Heterotopic Ossification Using Radiographic Imaging: Evidence for a Paradigm Shift
- PMID: 26544555
- PMCID: PMC4636348
- DOI: 10.1371/journal.pone.0141432
Characterization of Heterotopic Ossification Using Radiographic Imaging: Evidence for a Paradigm Shift
Abstract
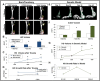
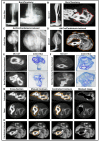
Heterotopic ossification (HO) is the growth of extra-skeletal bone which occurs following trauma, burns, and in patients with genetic bone morphogenetic protein (BMP) receptor mutations. The clinical and laboratory evaluation of HO is dependent on radiographic imaging to identify and characterize these lesions. Here we show that despite its inadequacies, plain film radiography and single modality microCT continue to serve as a primary method of HO imaging in nearly 30% of published in vivo literature. Furthermore, we demonstrate that detailed microCT analysis is superior to plain film and single modality microCT radiography specifically in the evaluation of HO formed through three representative models due to its ability to 1) define structural relationships between growing extra-skeletal bone and normal, anatomic bone, 2) provide accurate quantification and growth rate based on volume of the space-occupying lesion, thereby facilitating assessments of therapeutic intervention, 3) identify HO at earlier times allowing for evaluation of early intervention, and 4) characterization of metrics of bone physiology including porosity, tissue mineral density, and cortical and trabecular volume. Examination of our trauma model using microCT demonstrated two separate areas of HO based on anatomic location and relationship with surrounding, normal bone structures. Additionally, microCT allows HO growth rate to be evaluated to characterize HO progression. Taken together, these data demonstrate the need for a paradigm shift in the evaluation of HO towards microCT as a standard tool for imaging.
Conflict of interest statement
Figures



References
-
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006. 38: 525–527. - PubMed
-
- van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002. 40: 313–326. - PubMed
-
- Thomas BJ, Amstutz HC. Results of the administration of diphosphonate for the prevention of heterotopic ossification after total hip arthroplasty. J Bone Joint Surg Am. 1985. 67: 400–403. - PubMed
-
- Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001. 39: 370–374. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

