Merkel Cell Polyomavirus Small T Antigen Induces Cancer and Embryonic Merkel Cell Proliferation in a Transgenic Mouse Model
- PMID: 26544690
- PMCID: PMC4636375
- DOI: 10.1371/journal.pone.0142329
Merkel Cell Polyomavirus Small T Antigen Induces Cancer and Embryonic Merkel Cell Proliferation in a Transgenic Mouse Model
Abstract
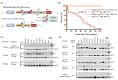
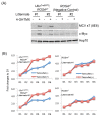
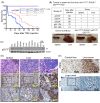
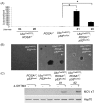
Merkel cell polyomavirus (MCV) causes the majority of human Merkel cell carcinomas (MCC) and encodes a small T (sT) antigen that transforms immortalized rodent fibroblasts in vitro. To develop a mouse model for MCV sT-induced carcinogenesis, we generated transgenic mice with a flox-stop-flox MCV sT sequence homologously recombined at the ROSA locus (ROSAsT), allowing Cre-mediated, conditional MCV sT expression. Standard tamoxifen (TMX) administration to adult UbcCreERT2; ROSAsT mice, in which Cre is ubiquitously expressed, resulted in MCV sT expression in multiple organs that was uniformly lethal within 5 days. Conversely, most adult UbcCreERT2; ROSAsT mice survived low-dose tamoxifen administration but developed ear lobe dermal hyperkeratosis and hypergranulosis. Simultaneous MCV sT expression and conditional homozygous p53 deletion generated multi-focal, poorly-differentiated, highly anaplastic tumors in the spleens and livers of mice after 60 days of TMX treatment. Mouse embryonic fibroblasts from these mice induced to express MCV sT exhibited anchorage-independent cell growth. To examine Merkel cell pathology, MCV sT expression was also induced during mid-embryogenesis in Merkel cells of Atoh1CreERT2/+; ROSAsT mice, which lead to significantly increased Merkel cell numbers in touch domes at late embryonic ages that normalized postnatally. Tamoxifen administration to adult Atoh1CreERT2/+; ROSAsT and Atoh1CreERT2/+; ROSAsT; p53flox/flox mice had no effects on Merkel cell numbers and did not induce tumor formation. Taken together, these results show that MCV sT stimulates progenitor Merkel cell proliferation in embryonic mice and is a bona fide viral oncoprotein that induces full cancer cell transformation in the p53-null setting.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

