The risk of viral rebound in the year after delivery in women remaining on antiretroviral therapy
- PMID: 26544700
- PMCID: PMC4631122
- DOI: 10.1097/QAD.0000000000000826
The risk of viral rebound in the year after delivery in women remaining on antiretroviral therapy
Abstract
Objective: The objective of this study is to assess the risk of viral rebound in postpartum women on suppressive combination antiretroviral therapy (cART).
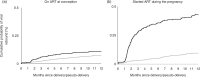
Methods: Using data from the UK Collaborative HIV Cohort (UK CHIC) study and the UK and Ireland National Study of HIV in Pregnancy and Childhood (NSHPC), women with HIV-RNA 50 copies/ml or less at delivery in 2006-2011, who started life-long cART during pregnancy (n = 321) or conceived on cART (n = 618), were matched by age, duration on cART and time period, with at least one control (non-postpartum). The cumulative probability of viral rebound (HIV-RNA >200 copies/ml) was assessed by Kaplan-Meier analysis; adjusted hazard ratios (aHRs) for the 0-3 and 3-12 months postdelivery (cases)/pseudo-delivery (controls) were calculated in Cox proportional hazards models.
Results: In postpartum women who conceived on cART, 5.9% [95% confidence interval (95% CI) 4.0-7.7] experienced viral rebound by 3 months, and 2.2% (1.4-3.0%) of their controls. The risk of viral rebound was higher in postpartum women than in controls during the first 3 months [aHR 2.63 (1.58-4.39)] but not during the 3-12 months postdelivery/pseudo-delivery. In postpartum women who started cART during pregnancy, 27% (22-32%) experienced viral rebound by 3 months, and 3.0% (1.6-4.4%) of their controls. The risk of viral rebound was higher in postpartum women than in controls during both postdelivery/pseudo-delivery periods [<3 months: aHR 6.63 (3.58-12.29); 3-12 months: aHR 4.05 (2.03-8.09)].
Conclusion: In women on suppressive cART, the risk of viral rebound is increased following delivery, especially in the first 3 months, which may be related to reduced adherence, indicating the need for additional adherence support for postpartum women.
Figures
References
-
- Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013). HIV Med 2014; 15 Suppl 1:1–85. - PubMed
-
- NSHPC. Routine NSHPC quarterly slides: slide 7. July 2014. www.nshpc.ucl.ac.uk [Accessed 25 July 2015]
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 30 June 2013. www.who.int/hiv/pub/guidelines/arv2013/download/en/ [Accessed 25 July 2015] - PubMed
-
- de Ruiter A, Taylor GP, Clayden P, Dhar J, Ghandi K, Gilleece Y, et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012 (2014 interim review). HIV Med 2014; 15 Suppl 4:1–77. - PubMed