Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats
- PMID: 26544861
- PMCID: PMC4636303
- DOI: 10.1371/journal.pone.0142424
Pretreatment with Resveratrol Prevents Neuronal Injury and Cognitive Deficits Induced by Perinatal Hypoxia-Ischemia in Rats
Abstract
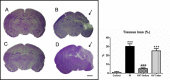
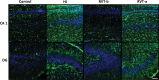
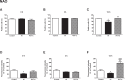
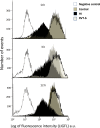
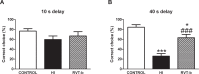
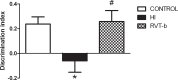
Despite advances in neonatal care, hypoxic-ischemic brain injury is still a serious clinical problem, which is responsible for many cases of perinatal mortality, cerebral palsy, motor impairment and cognitive deficits. Resveratrol, a natural polyphenol with important anti-oxidant and anti-inflammatory properties, is present in grapevines, peanuts and pomegranates. The aim of the present work was to evaluate the possible neuroprotective effect of resveratrol when administered before or immediately after a hypoxic-ischemic brain event in neonatal rats by analyzing brain damage, the mitochondrial status and long-term cognitive impairment. Our results indicate that pretreatment with resveratrol protects against brain damage, reducing infarct volume, preserving myelination and minimizing the astroglial reactive response. Moreover its neuroprotective effect was found to be long lasting, as behavioral outcomes were significantly improved at adulthood. We speculate that one of the mechanisms for this neuroprotection may be related to the maintenance of the mitochondrial inner membrane integrity and potential, and to the reduction of reactive oxygen species. Curiously, none of these protective features was observed when resveratrol was administered immediately after hypoxia-ischemia.
Conflict of interest statement
Figures







References
-
- du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol 2002. April;15(2):151–157. - PubMed
-
- Azra Haider B, Bhutta ZA. Birth asphyxia in developing countries: current status and public health implications. Curr Probl Pediatr Adolesc Health Care 2006. May-Jun;36(5):178–188. - PubMed
-
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 2001;7(1):56–64. - PubMed
-
- Low JA. Determining the contribution of asphyxia to brain damage in the neonate. J Obstet Gynaecol Res 2004. August;30(4):276–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

