Optimum population-level use of artemisinin combination therapies: a modelling study
- PMID: 26545449
- PMCID: PMC4641185
- DOI: 10.1016/S2214-109X(15)00162-X
Optimum population-level use of artemisinin combination therapies: a modelling study
Abstract
Background: Artemisinin combination therapies (ACTs) are used worldwide as first-line treatment against confirmed or suspected Plasmodium falciparum malaria. Despite the success of ACTs at reducing the global burden of malaria, emerging resistance to artemisinin threatens these gains. Countering onset of resistance might need deliberate tactics aimed at slowing the reduction in ACT effectiveness. We assessed optimum use of ACTs at the population level, specifically focusing on a strategy of multiple first-line therapies (MFT), and comparing it with strategies of cycling or sequential use of single first-line ACTs.
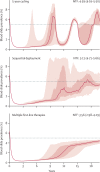
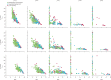
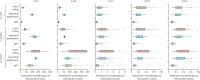
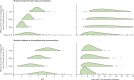
Methods: With an individual-based microsimulation of regional malaria transmission, we looked at how to apply a therapy as widely as possible without accelerating reduction of efficacy by drug resistance. We compared simultaneous distribution of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine (ie, MFT) against strategies in which these ACTs would be cycled or used sequentially, either on a fixed schedule or when population-level efficacy reaches the WHO threshold of 10% treatment failure. The main assessment criterion was total number of treatment failures per 100 people per year. Additionally, we analysed the benefits of including a single non-ACT therapy in an MFT strategy, and did sensitivity analyses in which we varied transmission setting, treatment coverage, partner-drug half-life, fitness cost of drug resistance, and the relation between drug concentration and resistance evolution.
Findings: Use of MFT was predicted to reduce the long-term number of treatment failures compared with strategies in which a single first-line ACT is recommended. This result was robust to various epidemiological, pharmacological, and evolutionary features of malaria transmission. Inclusion of a single non-ACT therapy in an MFT strategy would have substantial benefits in reduction of pressure on artemisinin resistance evolution, delaying its emergence and slowing its spread.
Interpretation: Adjusting national antimalarial treatment guidelines to encourage simultaneous use of MFT is likely to extend the useful therapeutic life of available antimalarial drugs, resulting in long-term beneficial outcomes for patients.
Funding: Wellcome Trust, UK Medical Research Council, Li Ka Shing Foundation.
Copyright © 2015 Copyright © Nguyen et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Saving lives through improved use of ACTs.Lancet Glob Health. 2015 Dec;3(12):e727-8. doi: 10.1016/S2214-109X(15)00208-9. Epub 2015 Nov 4. Lancet Glob Health. 2015. PMID: 26545448 No abstract available.
References
-
- WHO . Guidelines for the treatment of malaria. 1st edn. World Health Organization; Geneva: 2006.
-
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

