Mechanism Matters: A Taxonomy of Cell Penetrating Peptides
- PMID: 26545486
- PMCID: PMC4727446
- DOI: 10.1016/j.tibs.2015.10.004
Mechanism Matters: A Taxonomy of Cell Penetrating Peptides
Abstract
The permeability barrier imposed by cellular membranes limits the access of exogenous compounds to the interior of cells. Researchers and patients alike would benefit from efficient methods for intracellular delivery of a wide range of membrane-impermeant molecules, including biochemically active small molecules, imaging agents, peptides, peptide nucleic acids, proteins, RNA, DNA, and nanoparticles. There has been a sustained effort to exploit cell penetrating peptides (CPPs) for the delivery of such useful cargoes in vitro and in vivo because of their biocompatibility, ease of synthesis, and controllable physical chemistry. Here, we discuss the many mechanisms by which CPPs can function, and describe a taxonomy of mechanisms that could be help organize future efforts in the field.
Keywords: cell penetrating peptide (CPP); cytosolic delivery; membrane translocation; transient permeabilization.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


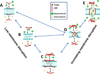
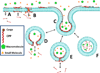
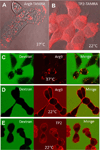
References
-
- Rydberg H, Matson M, Amand HL, Esbjarner EK, Norden B. Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry. 2012;51:5531–5539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

