Baseline characteristics, analysis plan and report on feasibility for the Prevention Of Decline in Cognition After Stroke Trial (PODCAST)
- PMID: 26545986
- PMCID: PMC4636808
- DOI: 10.1186/s13063-015-1033-2
Baseline characteristics, analysis plan and report on feasibility for the Prevention Of Decline in Cognition After Stroke Trial (PODCAST)
Abstract
Background: A common complication after stroke is development of cognitive impairment and dementia. However, effective strategies for reducing the risk of developing these problems remain undefined. Potential strategies include intensive lowering of blood pressure (BP) and/or lipids. This paper summarises the baseline characteristics, statistical analysis plan and feasibility of a randomised control trial of blood pressure and lipid lowering in patients post-stroke with the primary objective of reducing cognitive impairment and dementia.
Methods: The Prevention Of Decline in Cognition After Stroke Trial (PODCAST) was a multi-centre prospective randomised open-label blinded-endpoint controlled partial-factorial internal pilot trial running in secondary and primary care. Participants without dementia were enrolled 3-7 months post ischaemic stroke or spontaneous intracerebral haemorrhage, and randomised to intensive versus guideline BP lowering (target systolic BP <125 mmHg versus <140 mmHg); patients with ischaemic stroke were also randomised to intensive or guideline lipid lowering (target LDL cholesterol <1.4 mmol/L versus <3 mmol/L). The primary outcome was the Addenbrooke's Cognitive Examination-Revised; a key secondary outcome was to assess feasibility of performing a large trial of one or both interventions. Data are number (%) or mean (standard deviation). The trial was planned to last for 8 years with follow-up between 1 and 8 years. The plan for reporting the main results is included as Additional file 2.
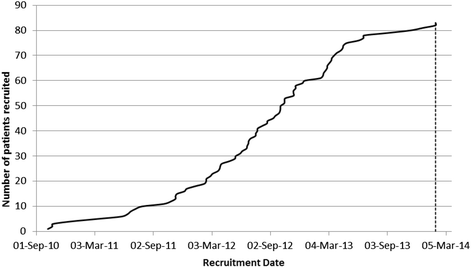
Results: 83 patients (of a planned 600) were recruited from 19 UK sites between 7 October 2010 and 31 January 2014. Delays, due to difficulties in the provision of excess treatment costs and to complexity of follow-up, led to few centres taking part and a much lower recruitment rate than planned. Patient characteristics at baseline were: age 74 (SD 7) years, male 64 (77 %), index stroke ischaemic 77 (93 %), stroke onset to randomisation 4.5 [SD 1.3] months, Addenbrooke's Cognitive Examination-Revised 86 (of 100, SD 8), Montreal Cognitive Assessment 24 (of 30, SD 3), BP 147/82 (SD 19/11) mmHg, total cholesterol 4.0 (SD 0.8) mmol/L and LDL cholesterol 2.0 (SD 0.7) mmol/L, modified Rankin Scale 1.1 (SD 0.8).
Conclusion: Limited recruitment suggests that a large trial is not feasible using the current protocol. The effects of the interventions on BP, lipids, and cognition will be reported in the main publication.
Trial registration: ISRCTN85562386 registered on 23 September 2009.
References
-
- EAFT (European Atrial Fibrillation Trial) Study Group Secondary prevention in non-rheumatic atrial fibrillation after TIA or minor stroke. Lancet. 1993;342:1255–62. - PubMed
-
- Leonardi-Bee J, Bath PM, Bousser MG, Davalos A, Diener H-C, Guiraud-Chaumeil B, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke. 2005;36(1):162–8. doi: 10.1161/01.STR.0000149621.95215.ea. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical