Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation
- PMID: 26546446
- PMCID: PMC4682305
- DOI: 10.1105/tpc.15.00605
Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-Like SMXL Repressor Proteins for Ubiquitination and Degradation
Abstract
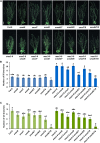
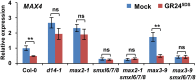
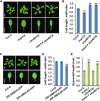
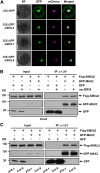
Strigolactones (SLs) are carotenoid-derived phytohormones that control many aspects of plant development, including shoot branching, leaf shape, stem secondary thickening, and lateral root growth. In rice (Oryza sativa), SL signaling requires the degradation of DWARF53 (D53), mediated by a complex including D14 and D3, but in Arabidopsis thaliana, the components and mechanism of SL signaling involving the D3 ortholog MORE AXILLARY GROWTH2 (MAX2) are unknown. Here, we show that SL-dependent regulation of shoot branching in Arabidopsis requires three D53-like proteins, SUPPRESSOR OF MORE AXILLARY GROWTH2-LIKE6 (SMXL6), SMXL7, and SMXL8. The smxl6 smxl7 smxl8 triple mutant suppresses the highly branched phenotypes of max2 and the SL-deficient mutant max3. Overexpression of a mutant form of SMXL6 that is resistant to SL-induced ubiquitination and degradation enhances shoot branching. Exogenous application of the SL analog rac-GR24 causes ubiquitination and degradation of SMXL6, 7, and 8; this requires D14 and MAX2. D53-like SMXLs form complexes with MAX2 and TOPLESS-RELATED PROTEIN2 (TPR2) and interact with D14 in a GR24-responsive manner. Furthermore, D53-like SMXLs exhibit TPR2-dependent transcriptional repression activity and repress the expression of BRANCHED1. Our findings reveal that in Arabidopsis, D53-like SMXLs act with TPR2 to repress transcription and so allow lateral bud outgrowth but that SL-induced degradation of D53-like proteins activates transcription to inhibit outgrowth.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Akiyama K., Matsuzaki K., Hayashi H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. - PubMed
-
- Al-Babili S., Bouwmeester H.J. (2015). Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 66: 161–186. - PubMed
-
- Alder A., Jamil M., Marzorati M., Bruno M., Vermathen M., Bigler P., Ghisla S., Bouwmeester H., Beyer P., Al-Babili S. (2012). The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

