SMAX1-LIKE/D53 Family Members Enable Distinct MAX2-Dependent Responses to Strigolactones and Karrikins in Arabidopsis
- PMID: 26546447
- PMCID: PMC4682302
- DOI: 10.1105/tpc.15.00562
SMAX1-LIKE/D53 Family Members Enable Distinct MAX2-Dependent Responses to Strigolactones and Karrikins in Arabidopsis
Abstract
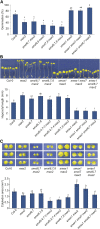
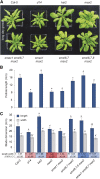
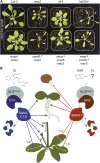
The plant hormones strigolactones and smoke-derived karrikins are butenolide signals that control distinct aspects of plant development. Perception of both molecules in Arabidopsis thaliana requires the F-box protein MORE AXILLARY GROWTH2 (MAX2). Recent studies suggest that the homologous SUPPRESSOR OF MAX2 1 (SMAX1) in Arabidopsis and DWARF53 (D53) in rice (Oryza sativa) are downstream targets of MAX2. Through an extensive analysis of loss-of-function mutants, we demonstrate that the Arabidopsis SMAX1-LIKE genes SMXL6, SMXL7, and SMXL8 are co-orthologs of rice D53 that promote shoot branching. SMXL7 is degraded rapidly after treatment with the synthetic strigolactone mixture rac-GR24. Like D53, SMXL7 degradation is MAX2- and D14-dependent and can be prevented by deletion of a putative P-loop. Loss of SMXL6,7,8 suppresses several other strigolactone-related phenotypes in max2, including increased auxin transport and PIN1 accumulation, and increased lateral root density. Although only SMAX1 regulates germination and hypocotyl elongation, SMAX1 and SMXL6,7,8 have complementary roles in the control of leaf morphology. Our data indicate that SMAX1 and SMXL6,7,8 repress karrikin and strigolactone signaling, respectively, and suggest that all MAX2-dependent growth effects are mediated by degradation of SMAX1/SMXL proteins. We propose that functional diversification within the SMXL family enabled responses to different butenolide signals through a shared regulatory mechanism.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures
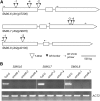
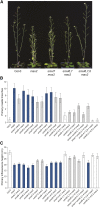
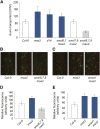


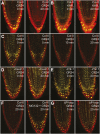


References
-
- Al-Babili S., Bouwmeester H.J. (2015). Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 66: 161–186. - PubMed
-
- Alder A., Jamil M., Marzorati M., Bruno M., Vermathen M., Bigler P., Ghisla S., Bouwmeester H., Beyer P., Al-Babili S. (2012). The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

