An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib
- PMID: 26546616
- PMCID: PMC4794361
- DOI: 10.1158/1078-0432.CCR-15-1588
An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib
Abstract
Purpose: To assess the tumor response to the smoothened (SMO) inhibitor, sonidegib (LDE225), in patients with an advanced basal cell carcinoma (BCC) resistant to treatment with vismodegib (GDC0449).
Experimental design: Nine patients with an advanced BCC that was previously resistant to treatment with vismodegib were given sonidegib in this investigational, open-label study. Tumor response was determined using the response evaluation criteria in solid tumors. SMO mutations were identified using biopsy samples from the target BCC location.
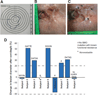
Results: The median duration of treatment with sonidegib was 6 weeks (range, 3-58 weeks). Five patients experienced progressive disease with sonidegib. Three patients experienced stable disease and discontinued sonidegib either due to adverse events (n = 1) or due to election for surgery (n = 2). The response of one patient was not evaluable. SMO mutations with in vitro data suggesting resistance to Hh pathway inhibition were identified in 5 patients, and none of these patients experienced responses while on sonidegib.
Conclusions: Patients with advanced BCCs that were previously resistant to treatment with vismodegib similarly demonstrated treatment resistance with sonidegib. Patients who have developed treatment resistance to an SMO inhibitor may continue to experience tumor progression in response to other SMO inhibitors.
©2015 American Association for Cancer Research.
Conflict of interest statement
A.L.S. Chang is a consultant/advisory board member for and reports receiving commercial research grants from Novartis. No potential conflicts of interest were disclosed by the other authors.
Figures
References
-
- Chang AL, Solomon JA, Hainsworth JD, Goldberg L, McKenna E, Day BM, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

