Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Drug-like Properties in Chronic Animal Model of Multiple Sclerosis
- PMID: 26546682
- PMCID: PMC4692201
- DOI: 10.1074/jbc.M115.679068
Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Drug-like Properties in Chronic Animal Model of Multiple Sclerosis
Abstract
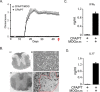
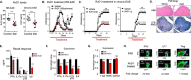
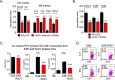
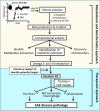
We performed untargeted metabolomics in plasma of B6 mice with experimental autoimmune encephalitis (EAE) at the chronic phase of the disease in search of an altered metabolic pathway(s). Of 324 metabolites measured, 100 metabolites that mapped to various pathways (mainly lipids) linked to mitochondrial function, inflammation, and membrane stability were observed to be significantly altered between EAE and control (p < 0.05, false discovery rate <0.10). Bioinformatics analysis revealed six metabolic pathways being impacted and altered in EAE, including α-linolenic acid and linoleic acid metabolism (PUFA). The metabolites of PUFAs, including ω-3 and ω-6 fatty acids, are commonly decreased in mouse models of multiple sclerosis (MS) and in patients with MS. Daily oral administration of resolvin D1, a downstream metabolite of ω-3, decreased disease progression by suppressing autoreactive T cells and inducing an M2 phenotype of monocytes/macrophages and resident brain microglial cells. This study provides a proof of principle for the application of metabolomics to identify an endogenous metabolite(s) possessing drug-like properties, which is assessed for therapy in preclinical mouse models of MS.
Keywords: autoimmune disease; inflammation; metabolism; metabolomics; multiple sclerosis; neuroinflammation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Miller J. R. (2004) The importance of early diagnosis of multiple sclerosis. J. Manag. Care Pharm. 10, S4–S11 - PubMed
-
- Lourenço A. S., Baldeiras I., Grãos M., and Duarte C. B. (2011) Proteomics-based technologies in the discovery of biomarkers for multiple sclerosis in the cerebrospinal fluid. Curr. Mol. Med. 11, 326–349 - PubMed
-
- Nicholson J. K., and Lindon J. C. (2008) Systems biology: metabonomics. Nature 455, 1054–1056 - PubMed
-
- Zhang A. H., Sun H., and Wang X. J. (2013) Recent advances in metabolomics in neurological disease, and future perspectives. Anal. Bioanal. Chem. 405, 8143–8150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

