Regulation of invadopodia by mechanical signaling
- PMID: 26546985
- PMCID: PMC4851576
- DOI: 10.1016/j.yexcr.2015.10.038
Regulation of invadopodia by mechanical signaling
Abstract
Mechanical rigidity in the tumor microenvironment is associated with a high risk of tumor formation and aggressiveness. Adhesion-based signaling driven by a rigid microenvironment is thought to facilitate invasion and migration of cancer cells away from primary tumors. Proteolytic degradation of extracellular matrix (ECM) is a key component of this process and is mediated by subcellular actin-rich structures known as invadopodia. Both ECM rigidity and cellular traction stresses promote invadopodia formation and activity, suggesting a role for these structures in mechanosensing. The presence and activity of mechanosensitive adhesive and signaling components at invadopodia further indicates the potential for these structures to utilize myosin-dependent forces to probe and remodel their ECM environments. Here, we provide a brief review of the role of adhesion-based mechanical signaling in controlling invadopodia and invasive cancer behavior.
Keywords: Actin; Adhesion; Contractility; Extracellular matrix; Invadopodia; Invasion; Mechanotransduction; Proteinases; Secretion; Signaling.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
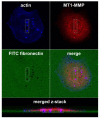
Similar articles
-
Polyacrylamide gels for invadopodia and traction force assays on cancer cells.J Vis Exp. 2015 Jan 4;(95):52343. doi: 10.3791/52343. J Vis Exp. 2015. PMID: 25590238 Free PMC article.
-
Extracellular matrix rigidity promotes invadopodia activity.Curr Biol. 2008 Sep 9;18(17):1295-1299. doi: 10.1016/j.cub.2008.07.090. Epub 2008 Aug 21. Curr Biol. 2008. PMID: 18718759 Free PMC article.
-
Cellular traction stresses mediate extracellular matrix degradation by invadopodia.Acta Biomater. 2014 May;10(5):1886-96. doi: 10.1016/j.actbio.2013.12.058. Epub 2014 Jan 8. Acta Biomater. 2014. PMID: 24412623 Free PMC article.
-
Biomechanical regulation of focal adhesion and invadopodia formation.J Cell Sci. 2020 Oct 22;133(20):jcs244848. doi: 10.1242/jcs.244848. J Cell Sci. 2020. PMID: 33093229 Review.
-
Signaling inputs to invadopodia and podosomes.J Cell Sci. 2013 Jul 15;126(Pt 14):2979-89. doi: 10.1242/jcs.079475. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843616 Free PMC article. Review.
Cited by
-
Invadopodia: clearing the way for cancer cell invasion.Ann Transl Med. 2020 Jul;8(14):902. doi: 10.21037/atm.2020.02.157. Ann Transl Med. 2020. PMID: 32793746 Free PMC article. Review.
-
Compression enhances invasive phenotype and matrix degradation of breast Cancer cells via Piezo1 activation.BMC Mol Cell Biol. 2022 Jan 3;23(1):1. doi: 10.1186/s12860-021-00401-6. BMC Mol Cell Biol. 2022. PMID: 34979904 Free PMC article.
-
Protrudin-mediated ER-endosome contact sites promote MT1-MMP exocytosis and cell invasion.J Cell Biol. 2020 Aug 3;219(8):e202003063. doi: 10.1083/jcb.202003063. J Cell Biol. 2020. PMID: 32479595 Free PMC article.
-
Stress-activated MAPKs and CRM1 regulate the subcellular localization of Net1A to control cell motility and invasion.J Cell Sci. 2018 Feb 1;131(3):jcs204644. doi: 10.1242/jcs.204644. J Cell Sci. 2018. PMID: 29361525 Free PMC article.
-
Integrins: Moonlighting Proteins in Invadosome Formation.Cancers (Basel). 2019 May 2;11(5):615. doi: 10.3390/cancers11050615. Cancers (Basel). 2019. PMID: 31052560 Free PMC article. Review.
References
-
- Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends in cell biology. 2007;17:178–186. - PubMed
-
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. Journal of mammary gland biology and neoplasia. 2004;9:325–342. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

