Synthesis of bi-phase dispersible core-shell FeAu@ZnO magneto-opto-fluorescent nanoparticles
- PMID: 26548369
- PMCID: PMC4637858
- DOI: 10.1038/srep16384
Synthesis of bi-phase dispersible core-shell FeAu@ZnO magneto-opto-fluorescent nanoparticles
Abstract
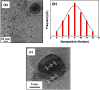
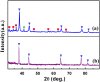
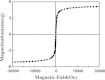
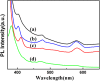
Bi-phase dispersible core-shell FeAu@ZnO magneto-opto-fluorescent nanoparticles were synthesized by a modified nanoemulsion process using poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEO-PPO-PEO) as the surfactant. The morphology and crystal structure of the nanoparticles were studied by TEM/HRTEM and XRD. The nanoparticles manifest soft ferromagnetic and/or near superparamagnetic behavior with a small coercivity of ~19 Oe at room temperature. The corresponding magnetic hysteresis curves were elucidated by the modified Langevin equation. The FTIR study confirms the PEO-PPO-PEO molecules on the surface of the nanoparticles. The UV-vis and PL results reveal the well-behaved absorption bands including surface plasmon resonance and multiple visible fingerprint photoluminescent emissions of the nanoparticles dispersed in both hydrophilic and hydrophobic solvents. Moreover, the processes of solvent dispersion-collection of the nanoparticles were demonstrated for application readiness of such core-shell nanostructures.
Figures




References
-
- Chiang I. C. & Chen D. H. Synthesis of Monodisperse FeAu Nanoparticles with Tunable Magnetic and Optical Properties. Adv. Funct. Mater. 17, 1311–1316 (2007).
-
- Mukherjee P. et al. Size-Induced Chemical and Magnetic Ordering in Individual Fe-Au Nanoparticles. ACS Nano 8, 8113–8120 (2014). - PubMed
-
- Amendola V. et al. Magneto-Plasmonic Au-Fe Alloy Nanoparticles Designed for Multimodal SERS-MRI-CT Imaging. Small. 10, 2476–2486 (2014). - PubMed
-
- Lee J. H. et al. Iron-Gold Barcode Nanowires. Angew. Chem. Int. Ed. 46, 3663–3667 (2007). - PubMed
-
- Amram D. & Rabkin E. Core (Fe)-Shell (Au) Nanoparticles Obtained from Thin Fe/Au Bilayers Employing Surface Segregation. ACS Nano 5, 10687–10693 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

