Synthesis and antileishmanial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives
- PMID: 26548988
- PMCID: PMC4970432
- DOI: 10.1186/s13588-014-0010-1
Synthesis and antileishmanial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives
Abstract
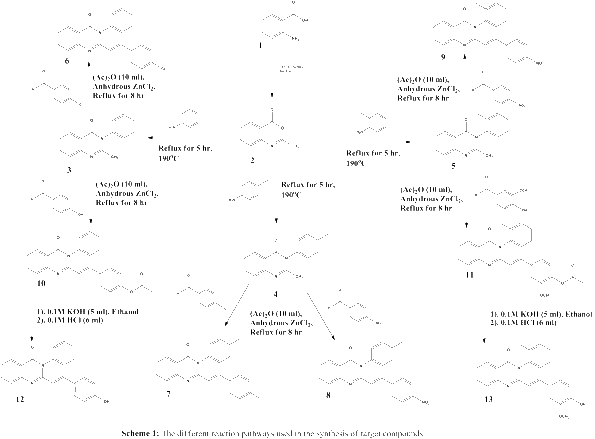
Background: Leishmaniasis is a neglected tropical parasitic diseases affecting millions of people around the globe. Quinazolines are a group of compounds with diverse pharmacological activities. Owing to their promising antileishmanial activities, some 3-aryl-2-(substitutedstyryl)-4(3H)-quinazolinones were synthesized in good yields (65.2% to 86.4%).
Results: The target compounds were synthesized by using cyclization, condensation, and hydrolysis reactions. The structures of the synthesized compounds were determined using elemental microanalysis, infrared (IR), and proton nuclear magnetic resonance ((1)H NMR). The in vitro antileishmanial activities of the synthesized compounds were evaluated using Leishmania donovani strain. All the synthesized compounds displayed appreciable antileishmanial activities (IC50 values, 0.0128 to 3.1085 μg/ml) as compared to the standard drug miltefosine (IC50 = 3.1911 μg/ml). (E)-2-(4-chlorostyryl)-3-p-tolyl-4(3H)-quinazolinone (7) is the compound with the most promising antileishmanial activities (IC50 = 0.0128 μg/ml) which is approximately 4 and 250 times more active than the standard drugs amphotericin B deoxycholate (IC50 = 0.0460 μg/ml) and miltefosine (IC50 = 3.1911 μg/ml), respectively.
Conclusions: The results obtained from this investigation indicate that the synthesized and biologically evaluated quinazoline compounds showed promising antileishmanial activities and are good scaffolds for the synthesis of different antileishmanial agents.
Keywords: Antileishmanial activities; Leishmania; Quinazolinones.
Figures
References
-
- Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008;45:255–272. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources