Interneuronal Nitric Oxide Signaling Mediates Post-synaptic Long-Term Depression of Striatal Glutamatergic Synapses
- PMID: 26549446
- PMCID: PMC4864038
- DOI: 10.1016/j.celrep.2015.10.015
Interneuronal Nitric Oxide Signaling Mediates Post-synaptic Long-Term Depression of Striatal Glutamatergic Synapses
Abstract
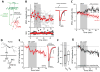
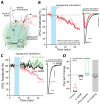
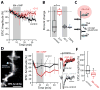
Experience-driven plasticity of glutamatergic synapses on striatal spiny projection neurons (SPNs) is thought to be essential to goal-directed behavior and habit formation. One major form of striatal plasticity, long-term depression (LTD), has long appeared to be expressed only pre-synaptically. Contrary to this view, nitric oxide (NO) generated by striatal interneurons was found to induce a post-synaptically expressed form of LTD at SPN glutamatergic synapses. This form of LTD was dependent on signaling through guanylyl cyclase and protein kinase G, both of which are abundantly expressed by SPNs. NO-LTD was unaffected by local synaptic activity or antagonism of endocannabinoid (eCb) and dopamine receptors, all of which modulate canonical, pre-synaptic LTD. Moreover, NO signaling disrupted induction of this canonical LTD by inhibiting dendritic Ca(2+) channels regulating eCb synthesis. These results establish an interneuron-dependent, heterosynaptic form of post-synaptic LTD that could act to promote stability of the striatal network during learning.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
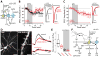
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Ariano MA. Distribution of components of the guanosine 3′,5′-phosphate system in rat caudate-putamen. Neuroscience. 1983;10:707–723. - PubMed
-
- Bender AT. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharmacological Reviews. 2006;58:488–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

