Telephone Assessment and Skill-Building Kit for Stroke Caregivers: A Randomized Controlled Clinical Trial
- PMID: 26549488
- PMCID: PMC4659731
- DOI: 10.1161/STROKEAHA.115.011099
Telephone Assessment and Skill-Building Kit for Stroke Caregivers: A Randomized Controlled Clinical Trial
Abstract
Background and purpose: There are few evidence-based programs for stroke family caregivers postdischarge. The purpose of this study was to evaluate efficacy of the Telephone Assessment and Skill-Building Kit (TASK II), a nurse-led intervention enabling caregivers to build skills based on assessment of their own needs.
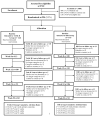
Methods: A total of 254 stroke caregivers (primarily female TASK II/information, support, and referral 78.0%/78.6%; white 70.7%/72.1%; about half spouses 48.4%/46.6%) were randomized to the TASK II intervention (n=123) or to an information, support, and referral group (n=131). Both groups received 8 weekly telephone sessions, with a booster at 12 weeks. General linear models with repeated measures tested efficacy, controlling for patient hospital days and call minutes. Prespecified 8-week primary outcomes were depressive symptoms (with Patient Health Questionnaire Depressive Symptom Scale PHQ-9 ≥5), life changes, and unhealthy days.
Results: Among caregivers with baseline PHQ-9 ≥5, those randomized to the TASK II intervention had a greater reduction in depressive symptoms from baseline to 8, 24, and 52 weeks and greater improvement in life changes from baseline to 12 weeks compared with the information, support, and referral group (P<0.05); but not found for the total sample. Although not sustained at 12, 24, or 52 weeks, caregivers randomized to the TASK II intervention had a relatively greater reduction in unhealthy days from baseline to 8 weeks (P<0.05).
Conclusions: The TASK II intervention reduced depressive symptoms and improved life changes for caregivers with mild to severe depressive symptoms. The TASK II intervention reduced unhealthy days for the total sample, although not sustained over the long term.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT01275495.
Keywords: caregivers; clinical trial; depression; psychology; stroke.
© 2015 American Heart Association, Inc.
Figures
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. - PubMed
-
- Bakas T, Austin JK, Okonkwo KF, Lewis RR, Chadwick LC. Needs, concerns, strategies, and advice of stroke caregivers the first 6 months after discharge. J Neurosci Nurs. 2002;34:242–251. - PubMed
-
- King RB, Semik PE. Stroke caregiving: difficult times, resource use, and needs during the first 2 years. J Gerontolog Nurs. 2006;32:37–44. - PubMed
-
- Quinn K, Murray C, Malone C. Spousal experiences of coping with and adapting to caregiving for a partner who has a stroke: a meta-synthesis of qualitative research. Disabil Rehabil. 2014;36:185–198. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous