NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine
- PMID: 26549640
- PMCID: PMC4667632
- DOI: 10.1038/ncomms9819
NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine
Erratum in
-
Author Correction: NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine.Nat Commun. 2020 Jun 10;11(1):3036. doi: 10.1038/s41467-020-16314-5. Nat Commun. 2020. PMID: 32522986 Free PMC article.
Abstract
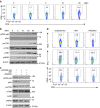
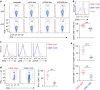
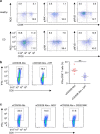
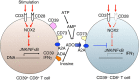
Interferon gamma (IFNγ)-producing CD8(+) T cells (Tc1) play important roles in immunological disease. We now report that CD3/CD28-mediated stimulation of CD8(+) T cells to generate Tc1 cells, not only increases IFNγ production but also boosts the generation of reactive oxygen species (ROS) and augments expression of CD39. Inhibition of NADPH oxidases or knockdown of gp91phox in CD8(+) T cells abrogates ROS generation, which in turn modulates JNK and NFκB signalling with decreases in both IFNγ levels and CD39 expression. CD39(+)CD8(+) T cells substantially inhibit IFNγ production by CD39(-)CD8(+) T cells via the paracrine generation of adenosine, which is operational via adenosine type 2A receptors. Increases in numbers of CD39(+)CD8(+) T cells and associated enhancements in ROS signal transduction are noted in cells from patients with Crohn's disease. Our findings provide insights into Tc1-mediated IFNγ responses and ROS generation and link these pathways to CD39/adenosine-mediated effects in immunological disease.
Figures




References
-
- Saurer L. & Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy 64, 505–519 (2009). - PubMed
-
- Brown S. J. & Mayer L. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 102, 2058–2069 (2007). - PubMed
-
- Geremia A., Biancheri P., Allan P., Corazza G. R. & Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13, 3–10 (2014). - PubMed
-
- Coskun M., Salem M., Pedersen J. & Nielsen O. H. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 76, 1–8 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

