NET formation can occur independently of RIPK3 and MLKL signaling
- PMID: 26549703
- PMCID: PMC4738457
- DOI: 10.1002/eji.201545615
NET formation can occur independently of RIPK3 and MLKL signaling
Abstract
The importance of neutrophil extracellular traps (NETs) in innate immunity is well established but the molecular mechanisms responsible for their formation are still a matter of scientific dispute. Here, we aim to characterize a possible role of the receptor-interacting protein kinase 3 (RIPK3) and the mixed lineage kinase domain-like (MLKL) signaling pathway, which are known to cause necroptosis, in NET formation. Using genetic and pharmacological approaches, we investigated whether this programmed form of necrosis is a prerequisite for NET formation. NETs have been defined as extracellular DNA scaffolds associated with the neutrophil granule protein elastase that are capable of killing bacteria. Neither Ripk3-deficient mouse neutrophils nor human neutrophils in which MLKL had been pharmacologically inactivated, exhibited abnormalities in NET formation upon physiological activation or exposure to low concentrations of PMA. These data indicate that NET formation occurs independently of both RIPK3 and MLKL signaling.
Keywords: MLKL; NET formation; NETosis; Necroptosis; Neutrophils; RIPK.
© 2015 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
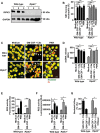
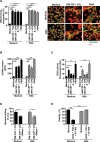
Comment in
-
Challenges in the characterization of neutrophil extracellular traps: The truth is in the details.Eur J Immunol. 2016 Jan;46(1):52-5. doi: 10.1002/eji.201546022. Eur J Immunol. 2016. PMID: 26635275
References
-
- Simon, D. , Simon, H. U. and Yousefi, S. , Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy 2013. 68: 409–416. - PubMed
-
- Brinkmann, V. , Reichard, U. , Goosmann, C. , Fauler, B. , Uhlemann, Y. , Weiss, D. S. , Weinrauch, Y. et al, Neutrophil extracellular traps kill bacteria. Science 2004. 303: 1532–1535. - PubMed
-
- Urban, C. F. , Reichard, U. , Brinkmann, V. and Zychlinsky, A. , Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006. 8: 668–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

