Molecular mechanisms regulating NLRP3 inflammasome activation
- PMID: 26549800
- PMCID: PMC4786634
- DOI: 10.1038/cmi.2015.95
Molecular mechanisms regulating NLRP3 inflammasome activation
Abstract
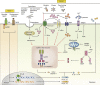
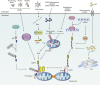
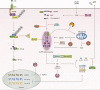
Inflammasomes are multi-protein signaling complexes that trigger the activation of inflammatory caspases and the maturation of interleukin-1β. Among various inflammasome complexes, the NLRP3 inflammasome is best characterized and has been linked with various human autoinflammatory and autoimmune diseases. Thus, the NLRP3 inflammasome may be a promising target for anti-inflammatory therapies. In this review, we summarize the current understanding of the mechanisms by which the NLRP3 inflammasome is activated in the cytosol. We also describe the binding partners of NLRP3 inflammasome complexes activating or inhibiting the inflammasome assembly. Our knowledge of the mechanisms regulating NLRP3 inflammasome signaling and how these influence inflammatory responses offers further insight into potential therapeutic strategies to treat inflammatory diseases associated with dysregulation of the NLRP3 inflammasome.
Figures
References
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002; 10: 417–426. - PubMed
-
- Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol 2011; 11: 213–220. - PubMed
-
- Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol 2011; 186: 2529–2534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

