CFTR Knockdown induces proinflammatory changes in intestinal epithelial cells
- PMID: 26549988
- PMCID: PMC4636765
- DOI: 10.1186/s12950-015-0107-y
CFTR Knockdown induces proinflammatory changes in intestinal epithelial cells
Abstract
Background: Hyperinflammation is a hallmark feature of cystic fibrosis (CF) airways. However, inflammation has also been documented systemically and, more recently, in extrapulmonary CF-affected tissues such as the pancreas and intestine. The pathogenesis of CF-related inflammation and more specifically the role of the cystic fibrosis transmembrane conductance regulator (CFTR) in that respect are not entirely understood. We have tested the hypothesis that genetic depletion of CFTR will affect the inflammatory status of human intestinal epithelial cell lines.
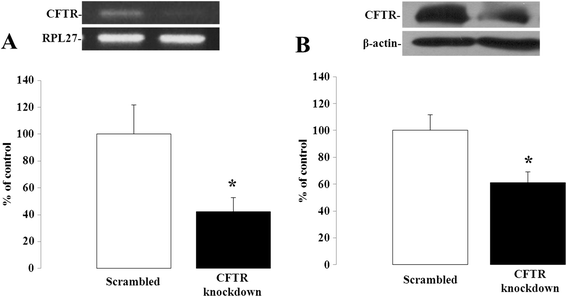
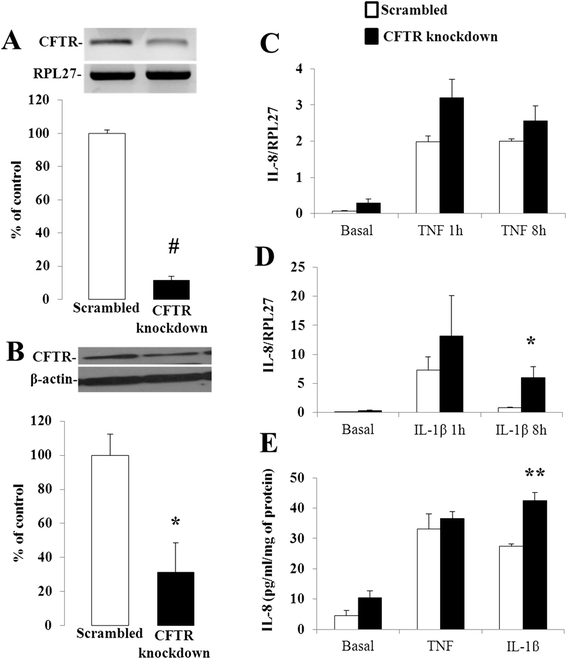
Methods: CFTR expression was genetically depleted from Caco-2/15 and HT-29 cells using short hairpin RNA interference (shRNAi). Inflammatory conditions were induced by the addition of human recombinant tumor necrosis factor (TNF) or Interleukin-1β (IL-1β) for various periods of time. Gene expression, mRNA stability and secreted levels of interleukin (IL)-6, -8 and 10 were assessed. Analysis of pro- and anti-inflammatory signaling pathways including mitogen-activated protein kinases (p38, ERK 1/2 and JNK), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα), and nuclear factor-kappa B (NF-κB) was also performed. Eosinophils were counted in the jejunal mucosa of Cftr-/- and Cftr+/+ mice.
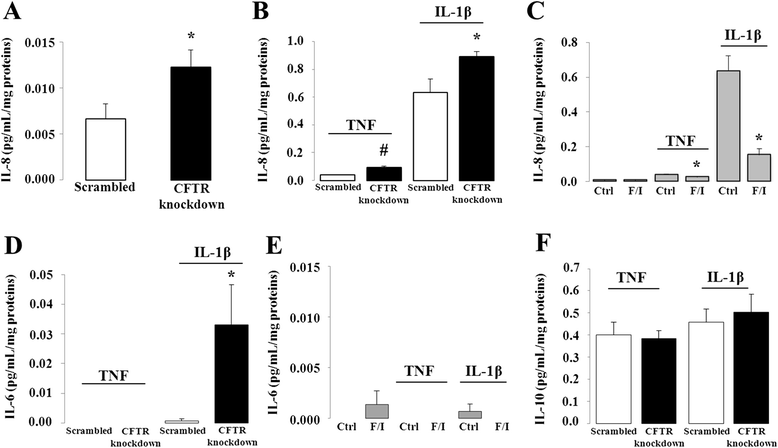
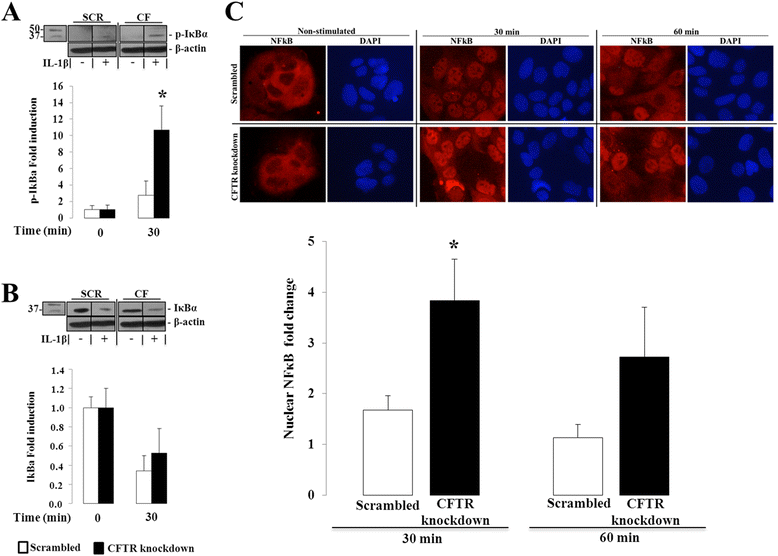
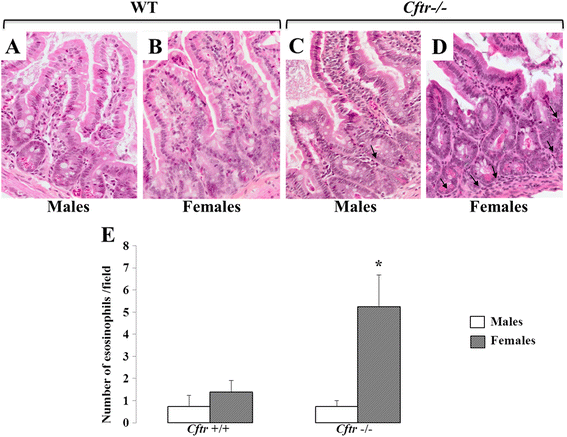
Results: CFTR gene and protein knockdown caused a significant increase in basal secretion of IL-8 as well as in IL-1β-induced secretion of IL-6 and -8. Release of the anti-inflammatory cytokine, IL-10, remained unaffected by CFTR depletion. The enhanced secretion of IL-8 stems in part from increased IL8 mRNA levels and greater activation of ERK1/2 MAPK, IκBα and NF-κB in the CFTR knockdown cells. By contrast, phosphorylation levels of p38 and JNK MAPK did not differ between control and knockdown cells. We also found a higher number of infiltrating eosinophils in the jejunal mucosa of Cftr -/- females, but not males, compared to Cftr +/+ mice, thus providing in vivo support to our in vitro findings.
Conclusion: Collectively, these data underscore the role played by CFTR in regulating the intestinal inflammatory responses. Such findings lend support to the theory that CFTR exerts functions that may go beyond its role as a chloride channel whereby its disruption may prevent cells to optimally respond to exogenous or endogenous challenges. These observations are of particular interest to CF patients who were found to display alterations in their intestinal microbiota, thus predisposing them to pathogens that may elicit exaggerated inflammatory responses.
Keywords: CFTR; Cystic fibrosis; Inflammation; Intestinal cell line.
Figures


References
-
- Cystic Fibrosis Foundation Patient Registry . 2013 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2014.
-
- Abu-El-Haija M, Ramachandran S, Meyerholz DK, Abu-El-Haija M, Griffin M, Giriyappa RL, Stoltz DA, Welsh MJ, McCray PB, Uc A. Pancreatic damage in fetal and newborn cystic fibrosis pigs involves the activation of inflammatory and remodeling pathways. Am J Pathol. 2012;181:499–507. doi: 10.1016/j.ajpath.2012.04.024. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

