Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts
- PMID: 26550143
- PMCID: PMC4612828
Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts
Abstract
Background: Curculigoside (CCG), one of the main bioactive phenolic compounds isolated from the rhizome of Curculigo orchioides Gaertn., is reported to prevent bone loss in ovariectomized rats. However, the underlying molecular mechanisms are largely unknown. Therefore, we investigated the effects of CCG on proliferation and differentiation of calvarial osteoblasts and discussed the related mechanisms.
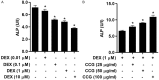
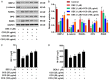
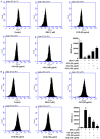
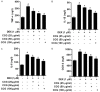
Materials and methods: Osteoblasts were incubated with dexamethasone (DEX) in the absence or presence of CCG concentrations for 24-72 h. Cell proliferation was evaluated by Cell Counting Kit-8 assay. Mitochondria membrane potential (MMP) and reactive oxygen species (ROS) were assessed by flow cytometry. We assessed the anti-inflammatory responses of CCG on DEX-induced osteoblasts by an enzyme-linked immunosorbent assay (ELISA). Relative protein expression of BMP-2, b-catenin, RANKL, OPG and RANK was measured using Western blotting.
Results: It was found that osteoblasts proliferation decreased significantly after treated with 1 μM of dexamethasone (DEX), compared with untreated osteoblasts and the cytotoxic effect of DEX was reversed remarkably when pretreatment with 25-100 μg/ml of CCG. Pretreatment with 25-100 μg/ml of CCG increased MMP level and decreased ROS production in osteoblasts induced by DEX. In addition, DEX-induced inhibition of differentiation markers such as alkaline phosphatase (ALP), OPG, BMP-2, β-catenin, IGF-1 and M-CSF level, and promotion of differentiation markers such as RANKL and RANK was significantly reversed in the presence of CCG. CCG also reversed DEX-induced production of pro-inflammatory cytokines.
Conclusions: These results provide new insights into the osteoblast-protective mechanisms of CCG through inducing proliferation and differentiation and reducing the inflammatory responses, indicating that CCG may be developed as an agent for the prevention and treatment of osteoporosis.
Keywords: Curculigoside; calvarial osteoblasts; osteoporosis; pro-inflammatory cytokines; proliferation.
Figures

Similar articles
-
Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts.Acta Biochim Biophys Sin (Shanghai). 2012 May;44(5):431-41. doi: 10.1093/abbs/gms014. Epub 2012 Mar 16. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22427460
-
Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway.Biomed Pharmacother. 2019 Feb;110:602-608. doi: 10.1016/j.biopha.2018.11.103. Epub 2018 Dec 8. Biomed Pharmacother. 2019. PMID: 30537677
-
Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells.Int J Mol Med. 2018 May;41(5):2517-2526. doi: 10.3892/ijmm.2018.3506. Epub 2018 Feb 20. Int J Mol Med. 2018. PMID: 29484386 Free PMC article.
-
Curculigoside promotes osteogenic differentiation of bone marrow stromal cells from ovariectomized rats.J Pharm Pharmacol. 2013 Jul;65(7):1005-13. doi: 10.1111/jphp.12054. Epub 2013 Apr 30. J Pharm Pharmacol. 2013. PMID: 23738728
-
Antiosteoporotic activity of phenolic compounds from Curculigo orchioides.Phytomedicine. 2009 Sep;16(9):874-81. doi: 10.1016/j.phymed.2009.01.005. Epub 2009 Mar 27. Phytomedicine. 2009. PMID: 19328665
Cited by
-
Network Pharmacology and Experimental Validation of the Therapeutic Effect of Baji Capsule on LPS-Induced Osteoporosis.Orthop Res Rev. 2025 Feb 11;17:61-81. doi: 10.2147/ORR.S488478. eCollection 2025. Orthop Res Rev. 2025. PMID: 39958436 Free PMC article.
-
Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis.Front Pharmacol. 2019 Nov 25;10:1344. doi: 10.3389/fphar.2019.01344. eCollection 2019. Front Pharmacol. 2019. PMID: 31824310 Free PMC article. Review.
-
Curculigoside Protects against Excess-Iron-Induced Bone Loss by Attenuating Akt-FoxO1-Dependent Oxidative Damage to Mice and Osteoblastic MC3T3-E1 Cells.Oxid Med Cell Longev. 2019 Dec 17;2019:9281481. doi: 10.1155/2019/9281481. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31949885 Free PMC article.
-
Phytochemistry and Pharmacological Activity of Plants of Genus Curculigo: An Updated Review Since 2013.Molecules. 2021 Jun 3;26(11):3396. doi: 10.3390/molecules26113396. Molecules. 2021. PMID: 34205154 Free PMC article. Review.
-
Effect of β‑ecdysterone on glucocorticoid‑induced apoptosis and autophagy in osteoblasts.Mol Med Rep. 2018 Jan;17(1):158-164. doi: 10.3892/mmr.2017.7840. Epub 2017 Oct 20. Mol Med Rep. 2018. PMID: 29115419 Free PMC article.
References
-
- Bilezikian JP. Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep. 2008;6:24–30. - PubMed
-
- Aswar U, Gurav M, More G, Rashed K, Aswar M. Effect of aqueous extract of Solanum xanthocarpum Schrad. & Wendl. on postmenopausal syndrome in ovariectomized rats. J Integr Med. 2014;5:439–446. - PubMed
-
- Tsai KY, Lee CC, Chou YM, Shen SP, Su CY, Wu HC, Huang MW, Shie JP, Chou FHC. The risks of major osteoporotic fractures in patients with schizophrenia: A population-based 10-year follow-up study. Schizophr Res. 2014;159:322–328. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
