Magnetic Resonance Imaging and Spectroscopy: Application to Experimental Neuro-Oncology
- PMID: 26550608
- PMCID: PMC4634890
Magnetic Resonance Imaging and Spectroscopy: Application to Experimental Neuro-Oncology
Abstract
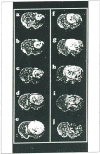
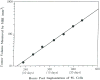
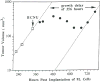
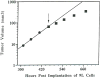
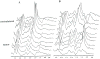
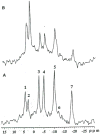
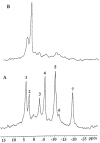
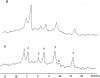
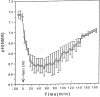
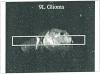
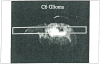
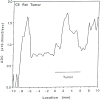
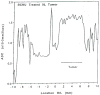
The development and use of animal brain tumor models over the past 25 years has helped to advance our understanding of both tumor biology and the effectiveness of new therapeutic approaches. The application of MRI and MRS as noninvasive tools for in vivo studies of intracerebral tumor models provides unique possibilities for furthering our knowledge of brain cancer. This article provides a brief background of traditional techniques used to evaluate growth and treatment efficacy in rodent brain tumor models and overviews the use of MR for quantitating intracerebral tumor growth kinetics and therapeutic response of experimental brain tumors from work conducted in this laboratory. The application of MRI and MRS in rodent brain tumor models for evaluation of novel therapeutic approaches, including gene transfer technology, is discussed. Finally, initial results with diffusion MRI for monitoring the treatment of brain tumors is introduced.
Keywords: 9L gliosarcoma; glioma; magnetic resonance imaging (MRI); magnetic resonance spectroscopy (MRS); tumor cell kill; tumor growth kinetics.
Figures

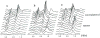





References
-
- Walker MD, Green SB, Byar DP, Alexander E, Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, Owens G, Ransohoff J, Robertson JT, Shapiro WR, Smith KR, Jr, Wilson CB, Strike TA. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1986;303:1323–1329. - PubMed
-
- Grossman SA, Burch PA. Quantitation of tumor response to antineoplastic therapy. Semin Oncol. 1988;15:441–454. - PubMed
-
- Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. - PubMed
-
- Wilson CB, Crafts D. Criteria of response and definition of recurrence. Natl Cancer Inst Monogr. 1977;46:197–203. - PubMed
-
- Grossman SA. Chemotherapy of brain tumors. In: Salcman M, editor. Concepts in neurosurgery. Vol. 4. Baltimore: Williams and Wilkins; 1991. pp. 321–340. (Neurobiology of brain tumors).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
