Both RIG-I and MDA5 detect alphavirus replication in concentration-dependent mode
- PMID: 26550947
- PMCID: PMC4721224
- DOI: 10.1016/j.virol.2015.09.023
Both RIG-I and MDA5 detect alphavirus replication in concentration-dependent mode
Abstract
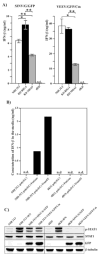
Alphaviruses are a family of positive-strand RNA viruses that circulate on all continents between mosquito vectors and vertebrate hosts. Despite a significant public health threat, their biology is not sufficiently investigated, and the mechanisms of alphavirus replication and virus-host interaction are insufficiently understood. In this study, we have applied a variety of experimental systems to further understand the mechanism by which infected cells detect replicating alphaviruses. Our new data strongly suggest that activation of the antiviral response by alphavirus-infected cells is determined by the integrity of viral genes encoding proteins with nuclear functions, and by the presence of two cellular pattern recognition receptors (PRRs), RIG-I and MDA5. No type I IFN response is induced in their absence. The presence of either of these PRRs is sufficient for detecting virus replication. However, type I IFN activation in response to pathogenic alphaviruses depends on the basal levels of RIG-I or MDA5.
Keywords: Alphaviruses; Innate immunity; MDA5; Pattern recognition receptors; RIG-I; Type I interferon; Virus–host interactions.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

