Erythropoietin Modulates Cerebral and Serum Degradation Products from Excess Calpain Activation following Prenatal Hypoxia-Ischemia
- PMID: 26551007
- PMCID: PMC4732905
- DOI: 10.1159/000441024
Erythropoietin Modulates Cerebral and Serum Degradation Products from Excess Calpain Activation following Prenatal Hypoxia-Ischemia
Abstract
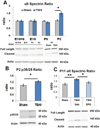
Preterm infants suffer central nervous system (CNS) injury from hypoxia-ischemia and inflammation - termed encephalopathy of prematurity. Mature CNS injury activates caspase and calpain proteases. Erythropoietin (EPO) limits apoptosis mediated by activated caspases, but its role in modulating calpain activation has not yet been investigated extensively following injury to the developing CNS. We hypothesized that excess calpain activation degrades developmentally regulated molecules essential for CNS circuit formation, myelination and axon integrity, including neuronal potassium-chloride co-transporter (KCC2), myelin basic protein (MBP) and phosphorylated neurofilament (pNF), respectively. Further, we predicted that post-injury EPO treatment could mitigate CNS calpain-mediated degradation. Using prenatal transient systemic hypoxia-ischemia (TSHI) in rats to mimic CNS injury from extreme preterm birth, and postnatal EPO treatment with a clinically relevant dosing regimen, we found sustained postnatal excess cortical calpain activation following prenatal TSHI, as shown by the cleavage of alpha II-spectrin (αII-spectrin) into 145-kDa αII-spectrin degradation products (αII-SDPs) and p35 into p25. Postnatal expression of the endogenous calpain inhibitor calpastatin was also reduced following prenatal TSHI. Calpain substrate expression following TSHI, including cortical KCC2, MBP and NF, was modulated by postnatal EPO treatment. Calpain activation was reflected in serum levels of αII-SDPs and KCC2 fragments, and notably, EPO treatment also modulated KCC2 fragment levels. Together, these data indicate that excess calpain activity contributes to the pathogenesis of encephalopathy of prematurity. Serum biomarkers of calpain activation may detect ongoing cerebral injury and responsiveness to EPO or similar neuroprotective strategies.
© 2015 S. Karger AG, Basel.
Conflict of interest statement
Figures




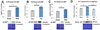
Similar articles
-
Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury.J Neurosurg Pediatr. 2016 Jun;17(6):739-55. doi: 10.3171/2015.10.PEDS15554. Epub 2016 Feb 19. J Neurosurg Pediatr. 2016. PMID: 26894518 Free PMC article.
-
Postnatal Erythropoietin Mitigates Impaired Cerebral Cortical Development Following Subplate Loss from Prenatal Hypoxia-Ischemia.Cereb Cortex. 2015 Sep;25(9):2683-95. doi: 10.1093/cercor/bhu066. Epub 2014 Apr 9. Cereb Cortex. 2015. PMID: 24722771 Free PMC article.
-
Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury.J Neurosurg Pediatr. 2010 Sep;6(3):206-21. doi: 10.3171/2010.5.PEDS1032. J Neurosurg Pediatr. 2010. PMID: 20809703 Free PMC article.
-
Erythropoietin for neonatal brain injury: opportunity and challenge.Int J Dev Neurosci. 2011 Oct;29(6):583-91. doi: 10.1016/j.ijdevneu.2010.12.007. Epub 2011 Jan 28. Int J Dev Neurosci. 2011. PMID: 21277366 Review.
-
Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors.Brain Res Brain Res Rev. 2003 May;42(2):169-85. doi: 10.1016/s0165-0173(03)00152-8. Brain Res Brain Res Rev. 2003. PMID: 12738057 Review.
Cited by
-
Extended Combined Neonatal Treatment With Erythropoietin Plus Melatonin Prevents Posthemorrhagic Hydrocephalus of Prematurity in Rats.Front Cell Neurosci. 2018 Sep 25;12:322. doi: 10.3389/fncel.2018.00322. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30319361 Free PMC article.
-
CXCR2 Blockade Mitigates Neural Cell Injury Following Preclinical Chorioamnionitis.Front Physiol. 2019 Apr 2;10:324. doi: 10.3389/fphys.2019.00324. eCollection 2019. Front Physiol. 2019. PMID: 31001130 Free PMC article.
-
Acute oligodendrocyte loss with persistent white matter injury in a third trimester equivalent mouse model of fetal alcohol spectrum disorder.Glia. 2017 Aug;65(8):1317-1332. doi: 10.1002/glia.23164. Epub 2017 May 18. Glia. 2017. PMID: 28518477 Free PMC article.
-
Imaging and serum biomarkers reflecting the functional efficacy of extended erythropoietin treatment in rats following infantile traumatic brain injury.J Neurosurg Pediatr. 2016 Jun;17(6):739-55. doi: 10.3171/2015.10.PEDS15554. Epub 2016 Feb 19. J Neurosurg Pediatr. 2016. PMID: 26894518 Free PMC article.
-
Neonatal administration of erythropoietin attenuates cognitive deficits in adult rats following placental insufficiency.J Neurosci Res. 2022 Dec;100(12):2112-2126. doi: 10.1002/jnr.24815. Epub 2021 Feb 20. J Neurosci Res. 2022. PMID: 33611820 Free PMC article.
References
-
- Wang KK. Calpain and caspase: can you tell the difference?, by kevin K.W. WangVol. 23, pp. 20–26. Trends Neurosci. 2000;23(2):59. - PubMed
-
- Chamnanvanakij S, Margraf LR, Burns D, Perlman JM. Apoptosis and white matter injury in preterm infants. Pediatr Dev Pathol. 2002;5(2):184–189. - PubMed
-
- Hargitai B, Szabo V, Hajdu J, Harmath A, Pataki M, Farid P, Papp Z, Szende B. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr Res. 2001;50(1):110–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

