Select Rab GTPases Regulate the Pulmonary Endothelium via Endosomal Trafficking of Vascular Endothelial-Cadherin
- PMID: 26551054
- PMCID: PMC4942219
- DOI: 10.1165/rcmb.2015-0286OC
Select Rab GTPases Regulate the Pulmonary Endothelium via Endosomal Trafficking of Vascular Endothelial-Cadherin
Abstract
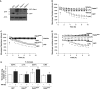
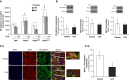
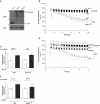
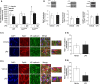
Pulmonary edema occurs in settings of acute lung injury, in diseases, such as pneumonia, and in acute respiratory distress syndrome. The lung interendothelial junctions are maintained in part by vascular endothelial (VE)-cadherin, an adherens junction protein, and its surface expression is regulated by endocytic trafficking. The Rab family of small GTPases are regulators of endocytic trafficking. The key trafficking pathways are regulated by Rab4, -7, and -9. Rab4 regulates the recycling of endosomes to the cell surface through a rapid-shuttle process, whereas Rab7 and -9 regulate trafficking to the late endosome/lysosome for degradation or from the trans-Golgi network to the late endosome, respectively. We recently demonstrated a role for the endosomal adaptor protein, p18, in regulation of the pulmonary endothelium through enhanced recycling of VE-cadherin to adherens junction. Thus, we hypothesized that Rab4, -7, and -9 regulate pulmonary endothelial barrier function through modulating trafficking of VE-cadherin-positive endosomes. We used Rab mutants with varying activities and associations to the endosome to study endothelial barrier function in vitro and in vivo. Our study demonstrates a key role for Rab4 activation and Rab9 inhibition in regulation of vascular permeability through enhanced VE-cadherin expression at the interendothelial junction. We further showed that endothelial barrier function mediated through Rab4 is dependent on extracellular signal-regulated kinase phosphorylation and activity. Thus, we demonstrate that Rab4 and -9 regulate VE-cadherin levels at the cell surface to modulate the pulmonary endothelium through extracellular signal-regulated kinase-dependent and -independent pathways, respectively. We propose that regulating select Rab GTPases represents novel therapeutic strategies for patients suffering with acute respiratory distress syndrome.
Keywords: Rab GTPase; acute respiratory distress syndrome; endocytosis; endothelium; vascular endothelial-cadherin.
Figures

Similar articles
-
p18, a novel adaptor protein, regulates pulmonary endothelial barrier function via enhanced endocytic recycling of VE-cadherin.FASEB J. 2015 Mar;29(3):868-81. doi: 10.1096/fj.14-257212. Epub 2014 Nov 17. FASEB J. 2015. PMID: 25404710 Free PMC article.
-
HIV-1 Nef Antagonizes SERINC5 Restriction by Downregulation of SERINC5 via the Endosome/Lysosome System.J Virol. 2018 May 14;92(11):e00196-18. doi: 10.1128/JVI.00196-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514909 Free PMC article.
-
Endolysosomal trafficking controls yolk granule biogenesis in vitellogenic Drosophila oocytes.PLoS Genet. 2024 Feb 5;20(2):e1011152. doi: 10.1371/journal.pgen.1011152. eCollection 2024 Feb. PLoS Genet. 2024. PMID: 38315726 Free PMC article.
-
ESCRTing the RABs through conversion.Biochem Soc Trans. 2025 Apr 30;53(2):431-445. doi: 10.1042/BST20253007. Biochem Soc Trans. 2025. PMID: 40605338 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Tyrosine phosphorylation of S1PR1 leads to chaperone BiP-mediated import to the endoplasmic reticulum.J Cell Biol. 2021 Nov 1;220(12):e202006021. doi: 10.1083/jcb.202006021. Epub 2021 Oct 15. J Cell Biol. 2021. PMID: 34652421 Free PMC article.
-
Pathophysiological Role of Vimentin Intermediate Filaments in Lung Diseases.Front Cell Dev Biol. 2022 Apr 28;10:872759. doi: 10.3389/fcell.2022.872759. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573702 Free PMC article. Review.
-
Activation of the sweet taste receptor, T1R3, by the artificial sweetener sucralose regulates the pulmonary endothelium.Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L165-L176. doi: 10.1152/ajplung.00490.2016. Epub 2017 Sep 28. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 28971978 Free PMC article.
-
Rab7a is required to degrade select blood-brain barrier junctional proteins after ischemic stroke.bioRxiv [Preprint]. 2025 May 20:2023.08.29.555373. doi: 10.1101/2023.08.29.555373. bioRxiv. 2025. PMID: 37693406 Free PMC article. Preprint.
-
Extracellular vesicles released from p18 overexpressing pulmonary endothelial cells are barrier protective - potential implications for acute respiratory distress syndrome.Pulm Circ. 2020 Sep 21;10(3):2045894020951759. doi: 10.1177/2045894020951759. eCollection 2020 Jul-Sep. Pulm Circ. 2020. PMID: 33014335 Free PMC article.
References
-
- Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986;133:913–927. - PubMed
-
- Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–L422. - PubMed
-
- Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113:1319–1334. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

