PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination
- PMID: 26551346
- PMCID: PMC4729458
- DOI: 10.1016/j.jconrel.2015.11.006
PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination
Abstract
The mucosa is the primary point of entry for pathogens making it an important vaccination site to produce a protective mucosal immune response. While the sublingual (SL) mucosa presents several barriers to vaccine penetration, its unique anatomy and physiology makes it one of the best options for mucosal vaccination. Efficient and directed delivery of adjuvants and antigens to appropriate immune mediators in the SL tissue will aid in development of effective SL vaccines against infectious diseases. Herein we demonstrate a robust immune response against influenza antigens co-delivered sublingually with engineered liposomes carrying the synthetic Toll-like receptor-4 agonist, CRX-601. Liposome modification with PEG copolymers (Pluronics), phospholipid-PEG conjugates and chitosan were evaluated for their ability to generate an immune response in a SL murine influenza vaccine model. Phospholipid-PEG conjugates were more effective than Pluronic copolymers in generating stable, surface neutral liposomes. SL vaccination with surface modified liposomes carrying CRX-601 adjuvant generated significant improvements in flu-specific responses compared with unmodified liposomes. Furthermore, the coating of modified liposomes with methylglycol chitosan produced the most effective flu-specific immune response. These results demonstrate efficient SL vaccine delivery utilizing a combination of a muco-adhesive and surface neutral liposomes to achieve a robust mucosal and systemic immune response.
Keywords: Chitosan; Influenza; Liposomes; Mucosal; Sublingual; TLR4; Vaccine.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

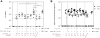
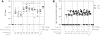


Similar articles
-
Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually.Vaccine. 2015 Oct 26;33(43):5845-5853. doi: 10.1016/j.vaccine.2015.08.086. Epub 2015 Sep 21. Vaccine. 2015. PMID: 26392012 Free PMC article.
-
A Lipopolysaccharide from Pantoea Agglomerans Is a Promising Adjuvant for Sublingual Vaccines to Induce Systemic and Mucosal Immune Responses in Mice via TLR4 Pathway.PLoS One. 2015 May 15;10(5):e0126849. doi: 10.1371/journal.pone.0126849. eCollection 2015. PLoS One. 2015. PMID: 25978818 Free PMC article.
-
A strong adjuvant based on glycol-chitosan-coated lipid-polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522.J Control Release. 2018 Feb 10;271:88-97. doi: 10.1016/j.jconrel.2017.12.003. Epub 2017 Dec 5. J Control Release. 2018. PMID: 29217176
-
The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza.Hum Vaccin Immunother. 2012 May;8(5):689-93. doi: 10.4161/hv.19568. Epub 2012 May 1. Hum Vaccin Immunother. 2012. PMID: 22495121 Review.
-
Biocompatible Mater Constructed Microneedle Arrays as a Novel Vaccine Adjuvant- Delivery System for Cutaneous and Mucosal Vaccination.Curr Pharm Des. 2015;21(36):5245-55. doi: 10.2174/1381612821666150923100147. Curr Pharm Des. 2015. PMID: 26412356 Review.
Cited by
-
Is the oral microbiome a source to enhance mucosal immunity against infectious diseases?NPJ Vaccines. 2021 Jun 2;6(1):80. doi: 10.1038/s41541-021-00341-4. NPJ Vaccines. 2021. PMID: 34078913 Free PMC article. Review.
-
Enabling sublingual peptide immunization with molecular self-assemblies.Biomaterials. 2020 May;241:119903. doi: 10.1016/j.biomaterials.2020.119903. Epub 2020 Feb 24. Biomaterials. 2020. PMID: 32143059 Free PMC article.
-
Brief on Recent Application of Liposomal Vaccines for Lower Respiratory Tract Viral Infections: From Influenza to COVID-19 Vaccines.Pharmaceuticals (Basel). 2021 Nov 17;14(11):1173. doi: 10.3390/ph14111173. Pharmaceuticals (Basel). 2021. PMID: 34832955 Free PMC article. Review.
-
Mucosal vaccine delivery: Current state and a pediatric perspective.J Control Release. 2016 Oct 28;240:394-413. doi: 10.1016/j.jconrel.2016.02.014. Epub 2016 Feb 6. J Control Release. 2016. PMID: 26860287 Free PMC article. Review.
-
Engineered Nanoparticle Applications for Recombinant Influenza Vaccines.Mol Pharm. 2021 Feb 1;18(2):576-592. doi: 10.1021/acs.molpharmaceut.0c00383. Epub 2020 Aug 17. Mol Pharm. 2021. PMID: 32787280 Free PMC article. Review.
References
-
- Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing Adjuvant Systems. Expert Rev Vaccines. 2011;10:471–486. - PubMed
-
- Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, Czerkinsky C, Kweon MN. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. Journal of immunology (Baltimore, Md : 1950) 2009;182:6851–6860. - PubMed
-
- Hervouet C, Luci C, Bekri S, Juhel T, Bihl F, Braud VM, Czerkinsky C, Anjuere F. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal immunology. 2014;7:280–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

