Potential therapeutic effects of mTOR inhibition in atherosclerosis
- PMID: 26551391
- PMCID: PMC5061792
- DOI: 10.1111/bcp.12820
Potential therapeutic effects of mTOR inhibition in atherosclerosis
Abstract
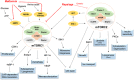
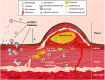
Despite significant improvement in the management of atherosclerosis, this slowly progressing disease continues to affect countless patients around the world. Recently, the mechanistic target of rapamycin (mTOR) has been identified as a pre-eminent factor in the development of atherosclerosis. mTOR is a constitutively active kinase found in two different multiprotein complexes, mTORC1 and mTORC2. Pharmacological interventions with a class of macrolide immunosuppressive drugs, called rapalogs, have shown undeniable evidence of the value of mTORC1 inhibition to prevent the development of atherosclerotic plaques in several animal models. Rapalog-eluting stents have also shown extraordinary results in humans, even though the exact mechanism for this anti-atherosclerotic effect remains elusive. Unfortunately, rapalogs are known to trigger diverse undesirable effects owing to mTORC1 resistance or mTORC2 inhibition. These adverse effects include dyslipidaemia and insulin resistance, both known triggers of atherosclerosis. Several strategies, such as combination therapy with statins and metformin, have been suggested to oppose rapalog-mediated adverse effects. Statins and metformin are known to inhibit mTORC1 indirectly via 5' adenosine monophosphate-activated protein kinase (AMPK) activation and may hold the key to exploit the full potential of mTORC1 inhibition in the treatment of atherosclerosis. Intermittent regimens and dose reduction have also been proposed to improve rapalog's mTORC1 selectivity, thereby reducing mTORC2-related side effects.
Keywords: atherosclerosis; mTOR; metformin; rapalogs; rapamycin.
© 2015 The British Pharmacological Society.
Figures

Similar articles
-
mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques.Atherosclerosis. 2014 Apr;233(2):601-607. doi: 10.1016/j.atherosclerosis.2014.01.040. Epub 2014 Jan 29. Atherosclerosis. 2014. PMID: 24534455 Review.
-
AZD2014, an Inhibitor of mTORC1 and mTORC2, Is Highly Effective in ER+ Breast Cancer When Administered Using Intermittent or Continuous Schedules.Mol Cancer Ther. 2015 Nov;14(11):2508-18. doi: 10.1158/1535-7163.MCT-15-0365. Epub 2015 Sep 10. Mol Cancer Ther. 2015. PMID: 26358751
-
Molecular regulation of apoptotic machinery and lipid metabolism by mTORC1/mTORC2 dual inhibitors in preclinical models of HER2+/PIK3CAmut breast cancer.Oncotarget. 2016 Oct 11;7(41):67071-67086. doi: 10.18632/oncotarget.11490. Oncotarget. 2016. PMID: 27563814 Free PMC article.
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
Cited by
-
Adropin inhibits the phenotypic modulation and proliferation of vascular smooth muscle cells during neointimal hyperplasia by activating the AMPK/ACC signaling pathway.Exp Ther Med. 2021 Jun;21(6):560. doi: 10.3892/etm.2021.9992. Epub 2021 Mar 26. Exp Ther Med. 2021. PMID: 33850532 Free PMC article.
-
mTOR Inhibitor Therapy and Metabolic Consequences: Where Do We Stand?Oxid Med Cell Longev. 2018 Jun 24;2018:2640342. doi: 10.1155/2018/2640342. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30034573 Free PMC article. Review.
-
Combined application of rapamycin and atorvastatin improves lipid metabolism in apolipoprotein E-deficient mice with chronic kidney disease.BMB Rep. 2021 Mar;54(3):170-175. doi: 10.5483/BMBRep.2021.54.3.136. BMB Rep. 2021. PMID: 33050984 Free PMC article.
-
A New Hypothesis Describing the Pathogenesis of Oral Mucosal Injury Associated with the Mammalian Target of Rapamycin (mTOR) Inhibitors.Cancers (Basel). 2023 Dec 22;16(1):68. doi: 10.3390/cancers16010068. Cancers (Basel). 2023. PMID: 38201496 Free PMC article.
-
Rapamycin in Cerebral Cavernous Malformations: What Doses to Test in Mice and Humans.ACS Pharmacol Transl Sci. 2022 Apr 25;5(5):266-277. doi: 10.1021/acsptsci.2c00006. eCollection 2022 May 13. ACS Pharmacol Transl Sci. 2022. PMID: 35592432 Free PMC article. Review.
References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–292. - PMC - PubMed
-
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011; 17: 1410–22. - PubMed
-
- Vanepps JS, Vorp DA. Mechano‐pathobiology of atherogenesis: a review. J Surg Res 2007; 142: 202–17. - PubMed
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473: 317–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

