Parcellating cortical functional networks in individuals
- PMID: 26551545
- PMCID: PMC4661084
- DOI: 10.1038/nn.4164
Parcellating cortical functional networks in individuals
Abstract
The capacity to identify the unique functional architecture of an individual's brain is a crucial step toward personalized medicine and understanding the neural basis of variation in human cognition and behavior. Here we developed a cortical parcellation approach to accurately map functional organization at the individual level using resting-state functional magnetic resonance imaging (fMRI). A population-based functional atlas and a map of inter-individual variability were employed to guide the iterative search for functional networks in individual subjects. Functional networks mapped by this approach were highly reproducible within subjects and effectively captured the variability across subjects, including individual differences in brain lateralization. The algorithm performed well across different subject populations and data types, including task fMRI data. The approach was then validated by invasive cortical stimulation mapping in surgical patients, suggesting potential for use in clinical applications.
Conflict of interest statement
H.L., D.W., R.L.B., and M.D.F are listed as inventors on submitted patents on mapping functional brain organization using fMRI. M.D.F. is listed as inventor on submitted or issued patents on guiding noninvasive brain stimulation with fMRI.
Figures


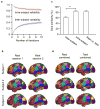
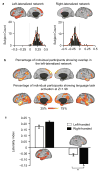
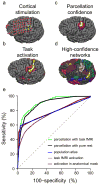
References
-
- Brodmann K. In: Localisation in the cerebral cortex. Garey LJ, translator. New York: Springer; 1909/2006.
-
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:292–398.
-
- Zilles K, Amunts K. Centenary of Brodmann’s map—conception and fate. Nat Rev Neurosci. 2010;11:139–145. - PubMed
-
- Amunts K, et al. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 MH106435/MH/NIMH NIH HHS/United States
- K23 NS083741/NS/NINDS NIH HHS/United States
- R01 NS069696/NS/NINDS NIH HHS/United States
- K23 MH104515/MH/NIMH NIH HHS/United States
- P50MH106435/MH/NIMH NIH HHS/United States
- R01 NS091604/NS/NINDS NIH HHS/United States
- K01MH099232/MH/NIMH NIH HHS/United States
- K25 NS069805/NS/NINDS NIH HHS/United States
- K25NS069805/NS/NINDS NIH HHS/United States
- R01 HD067312/HD/NICHD NIH HHS/United States
- 1U54MH091657/MH/NIMH NIH HHS/United States
- P41EB015902/EB/NIBIB NIH HHS/United States
- R01NS091604/NS/NINDS NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- K01 MH099232/MH/NIMH NIH HHS/United States
- P41 EB015902/EB/NIBIB NIH HHS/United States
- R01HD067312/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

