Platelet-derived HMGB1 is a critical mediator of thrombosis
- PMID: 26551681
- PMCID: PMC4665785
- DOI: 10.1172/JCI81660
Platelet-derived HMGB1 is a critical mediator of thrombosis
Abstract
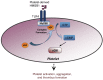
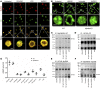
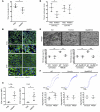
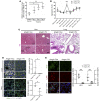
Thrombosis and inflammation are intricately linked in several major clinical disorders, including disseminated intravascular coagulation and acute ischemic events. The damage-associated molecular pattern molecule high-mobility group box 1 (HMGB1) is upregulated by activated platelets in multiple inflammatory diseases; however, the contribution of platelet-derived HMGB1 in thrombosis remains unexplored. Here, we generated transgenic mice with platelet-specific ablation of HMGB1 and determined that platelet-derived HMGB1 is a critical mediator of thrombosis. Mice lacking HMGB1 in platelets exhibited increased bleeding times as well as reduced thrombus formation, platelet aggregation, inflammation, and organ damage during experimental trauma/hemorrhagic shock. Platelets were the major source of HMGB1 within thrombi. In trauma patients, HMGB1 expression on the surface of circulating platelets was markedly upregulated. Moreover, evaluation of isolated platelets revealed that HMGB1 is critical for regulating platelet activation, granule secretion, adhesion, and spreading. These effects were mediated via TLR4- and MyD88-dependent recruitment of platelet guanylyl cyclase (GC) toward the plasma membrane, followed by MyD88/GC complex formation and activation of the cGMP-dependent protein kinase I (cGKI). Thus, we establish platelet-derived HMGB1 as an important mediator of thrombosis and identify a HMGB1-driven link between MyD88 and GC/cGKI in platelets. Additionally, these findings suggest a potential therapeutic target for patients sustaining trauma and other inflammatory disorders associated with abnormal coagulation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

