Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study
- PMID: 26552054
- PMCID: PMC4803028
- DOI: 10.1001/jamapediatrics.2015.2757
Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study
Abstract
Importance: Probiotics have been hypothesized to affect immunologic responses to environmental exposures by supporting healthy gut microbiota and could therefore theoretically be used to prevent the development of type 1 diabetes mellitus (T1DM)-associated islet autoimmunity.
Objective: To examine the association between supplemental probiotic use during the first year of life and islet autoimmunity among children at increased genetic risk of T1DM.
Design, setting, and participants: In this ongoing prospective cohort study that started September 1, 2004, children from 6 clinical centers, 3 in the United States (Colorado, Georgia/Florida, and Washington) and 3 in Europe (Finland, Germany, and Sweden), were followed up for T1DM-related autoantibodies. Blood samples were collected every 3 months between 3 and 48 months of age and every 6 months thereafter to determine persistent islet autoimmunity. Details of infant feeding, including probiotic supplementation and infant formula use, were monitored from birth using questionnaires and diaries. We applied time-to-event analysis to study the association between probiotic use and islet autoimmunity, stratifying by country and adjusting for family history of type 1 diabetes, HLA-DR-DQ genotypes, sex, birth order, mode of delivery, exclusive breastfeeding, birth year, child's antibiotic use, and diarrheal history, as well as maternal age, probiotic use, and smoking. Altogether 8676 infants with an eligible genotype were enrolled in the follow-up study before the age of 4 months. The final sample consisted of 7473 children with the age range of 4 to 10 years (as of October 31, 2014).
Exposures: Early intake of probiotics.
Main outcomes and measures: Islet autoimmunity revealed by specific islet autoantibodies.
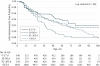
Results: Early probiotic supplementation (at the age of 0-27 days) was associated with a decreased risk of islet autoimmunity when compared with probiotic supplementation after 27 days or no probiotic supplementation (hazard ratio [HR], 0.66; 95% CI, 0.46-0.94). The association was accounted for by children with the DR3/4 genotype (HR, 0.40; 95% CI, 0.21-0.74) and was absent among other genotypes (HR, 0.97; 95% CI, 0.62-1.54).
Conclusions and relevance: Early probiotic supplementation may reduce the risk of islet autoimmunity in children at the highest genetic risk of T1DM. The result needs to be confirmed in further studies before any recommendation of probiotics use is made.
Figures
Comment in
-
A Glimpse of Microbial Power in Preventive Medicine.JAMA Pediatr. 2016 Jan;170(1):11. doi: 10.1001/jamapediatrics.2015.3246. JAMA Pediatr. 2016. PMID: 26551837 No abstract available.
References
-
- Hara N, Alkanani AK, Ir D, et al. The role of the intestinal microbiota in type 1 diabetes. Clin Immunol. 2013;146(2):112–119. - PubMed
-
- Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69(6):465–472. - PubMed
-
- Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–120. doi:10.1038/cmi. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK95300/DK/NIDDK NIH HHS/United States
- U01 DK63861/DK/NIDDK NIH HHS/United States
- UC4 DK63863/DK/NIDDK NIH HHS/United States
- U01 DK63821/DK/NIDDK NIH HHS/United States
- HHSN267200700014C/DK/NIDDK NIH HHS/United States
- UC4 DK63865/DK/NIDDK NIH HHS/United States
- U01 DK063861/DK/NIDDK NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- U01 DK063790/DK/NIDDK NIH HHS/United States
- U01 DK63829/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UC4 DK63829/DK/NIDDK NIH HHS/United States
- U01 DK63836/DK/NIDDK NIH HHS/United States
- UC4 DK63821/DK/NIDDK NIH HHS/United States
- UC4 DK063863/DK/NIDDK NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UC4 DK63836/DK/NIDDK NIH HHS/United States
- U01 DK063836/DK/NIDDK NIH HHS/United States
- U01 DK063829/DK/NIDDK NIH HHS/United States
- U01 DK063865/DK/NIDDK NIH HHS/United States
- UC4 DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063861/DK/NIDDK NIH HHS/United States
- U01 DK63865/DK/NIDDK NIH HHS/United States
- UC4 DK063829/DK/NIDDK NIH HHS/United States
- UC4 DK063821/DK/NIDDK NIH HHS/United States
- UC4 DK117483/DK/NIDDK NIH HHS/United States
- UC4 DK063836/DK/NIDDK NIH HHS/United States
- UC4 DK112243/DK/NIDDK NIH HHS/United States
- U01 DK063863/DK/NIDDK NIH HHS/United States
- U01 DK63790/DK/NIDDK NIH HHS/United States
- UC4 DK063865/DK/NIDDK NIH HHS/United States
- UC4 DK106955/DK/NIDDK NIH HHS/United States
- UC4 DK100238/DK/NIDDK NIH HHS/United States
- U01 DK63863/DK/NIDDK NIH HHS/United States
- UC4 DK63861/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials