Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer
- PMID: 26552413
- PMCID: PMC4707138
- DOI: 10.1158/2159-8290.CD-15-0023
Lymphatic Vessels, Inflammation, and Immunity in Skin Cancer
Abstract
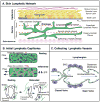
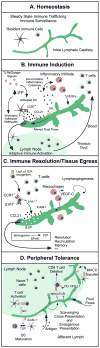
Skin is a highly ordered immune organ that coordinates rapid responses to external insult while maintaining self-tolerance. In healthy tissue, lymphatic vessels drain fluid and coordinate local immune responses; however, environmental factors induce lymphatic vessel dysfunction, leading to lymph stasis and perturbed regional immunity. These same environmental factors drive the formation of local malignancies, which are also influenced by local inflammation. Herein, we discuss clinical and experimental evidence supporting the tenet that lymphatic vessels participate in regulation of cutaneous inflammation and immunity, and are important contributors to malignancy and potential biomarkers and targets for immunotherapy.
Significance: The tumor microenvironment and tumor-associated inflammation are now appreciated not only for their role in cancer progression but also for their response to therapy. The lymphatic vasculature is a less-appreciated component of this microenvironment that coordinates local inflammation and immunity and thereby critically shapes local responses. A mechanistic understanding of the complexities of lymphatic vessel function in the unique context of skin provides a model to understand how regional immune dysfunction drives cutaneous malignancies, and as such lymphatic vessels represent a biomarker of cutaneous immunity that may provide insight into cancer prognosis and effective therapy.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

Similar articles
-
Lymphatic vessels regulate immune microenvironments in human and murine melanoma.J Clin Invest. 2016 Sep 1;126(9):3389-402. doi: 10.1172/JCI79434. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525437 Free PMC article.
-
Intralymphatic Spread Is a Common Finding in Cutaneous CD30+ Lymphoproliferative Disorders.Am J Surg Pathol. 2015 Nov;39(11):1511-7. doi: 10.1097/PAS.0000000000000474. Am J Surg Pathol. 2015. PMID: 26371781
-
The lymphatic vasculature: development and role in shaping immunity.Immunol Rev. 2016 May;271(1):276-92. doi: 10.1111/imr.12413. Immunol Rev. 2016. PMID: 27088921 Review.
-
Role of lymphatic vessels in tumor immunity: passive conduits or active participants?J Mammary Gland Biol Neoplasia. 2010 Sep;15(3):341-52. doi: 10.1007/s10911-010-9193-x. Epub 2010 Sep 11. J Mammary Gland Biol Neoplasia. 2010. PMID: 20835756 Review.
-
The VEGF-C/VEGFR3 signaling pathway contributes to resolving chronic skin inflammation by activating lymphatic vessel function.J Dermatol Sci. 2014 Feb;73(2):135-41. doi: 10.1016/j.jdermsci.2013.10.006. Epub 2013 Oct 29. J Dermatol Sci. 2014. PMID: 24252749
Cited by
-
IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin.J Exp Med. 2018 Dec 3;215(12):3057-3074. doi: 10.1084/jem.20180654. Epub 2018 Oct 31. J Exp Med. 2018. PMID: 30381467 Free PMC article.
-
Epidermal FABP Prevents Chemical-Induced Skin Tumorigenesis by Regulation of TPA-Induced IFN/p53/SOX2 Pathway in Keratinocytes.J Invest Dermatol. 2018 Sep;138(9):1925-1934. doi: 10.1016/j.jid.2018.02.041. Epub 2018 Mar 17. J Invest Dermatol. 2018. PMID: 29559340 Free PMC article.
-
Single-cell transcriptomics of melanoma sentinel lymph nodes identifies immune cell signatures associated with metastasis.JCI Insight. 2025 Mar 6;10(7):e183080. doi: 10.1172/jci.insight.183080. JCI Insight. 2025. PMID: 40048259 Free PMC article.
-
Single and combined impacts of irradiation and surgery on lymphatic vasculature and fibrosis associated to secondary lymphedema.Front Pharmacol. 2022 Oct 18;13:1016138. doi: 10.3389/fphar.2022.1016138. eCollection 2022. Front Pharmacol. 2022. PMID: 36330083 Free PMC article.
-
The Interplay Between Lymphatic Vessels and Chemokines.Front Immunol. 2019 Apr 12;10:518. doi: 10.3389/fimmu.2019.00518. eCollection 2019. Front Immunol. 2019. PMID: 31105685 Free PMC article. Review.
References
-
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014 Apr 11;14(5):289–301. - PubMed
-
- Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol. 1983 Jun;80( Suppl):12s– 16s. - PubMed
-
- Gajewski TF, Woo S-R, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013 Apr;25(2):268–76. - PubMed
-
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011 Oct;11(10):702–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

