Molecular insights into the surface-specific arrangement of complement C5 convertase enzymes
- PMID: 26552476
- PMCID: PMC4638095
- DOI: 10.1186/s12915-015-0203-8
Molecular insights into the surface-specific arrangement of complement C5 convertase enzymes
Abstract
Background: Complement is a large protein network in plasma that is crucial for human immune defenses and a major cause of aberrant inflammatory reactions. The C5 convertase is a multi-molecular protease complex that catalyses the cleavage of native C5 into its biologically important products. So far, it has been difficult to study the exact molecular arrangement of C5 convertases, because their non-catalytic subunits (C3b) are covalently linked to biological surfaces through a reactive thioester. Through development of a highly purified model system for C5 convertases, we here aim to provide insights into the surface-specific nature of these important protease complexes.
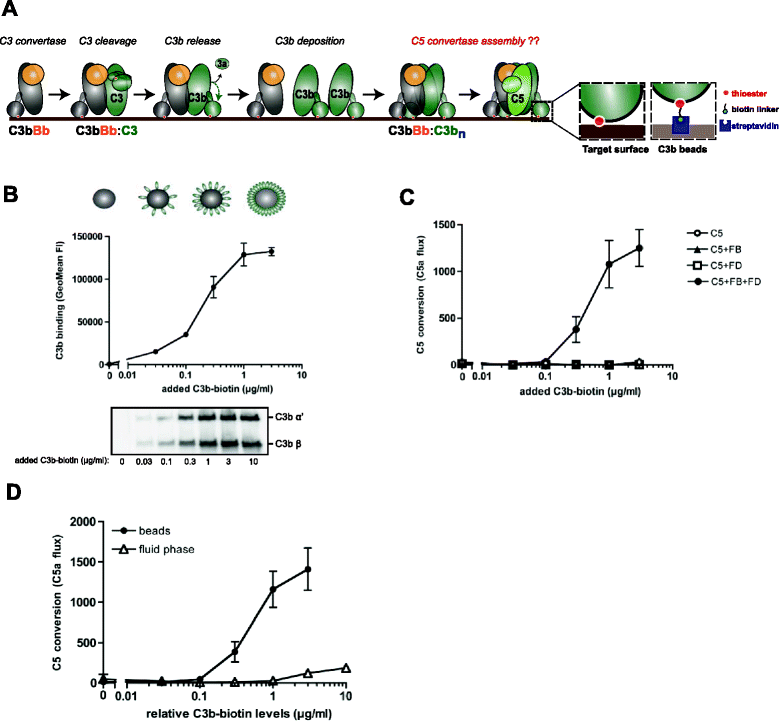
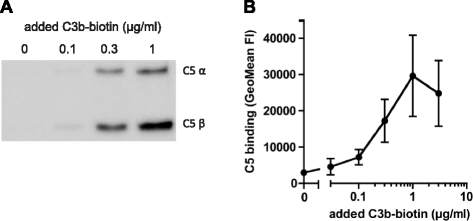
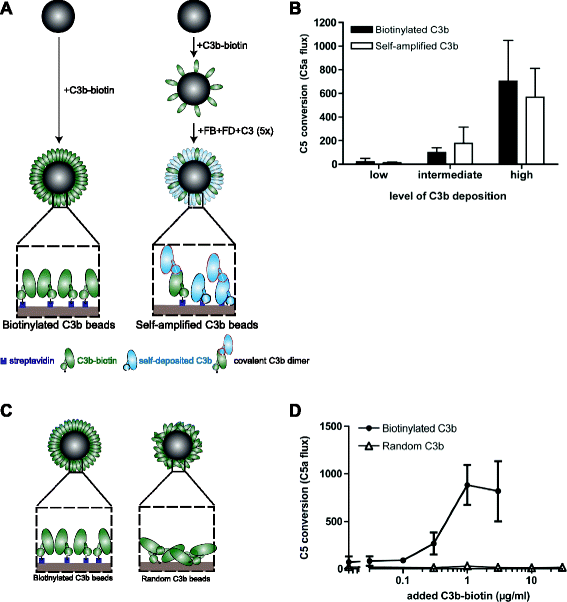
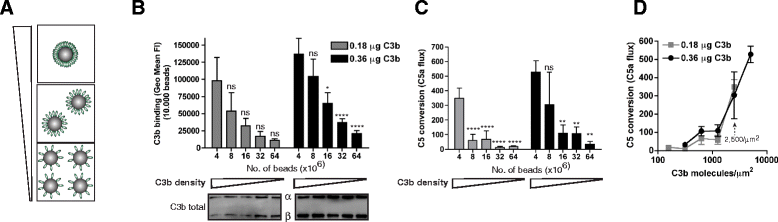
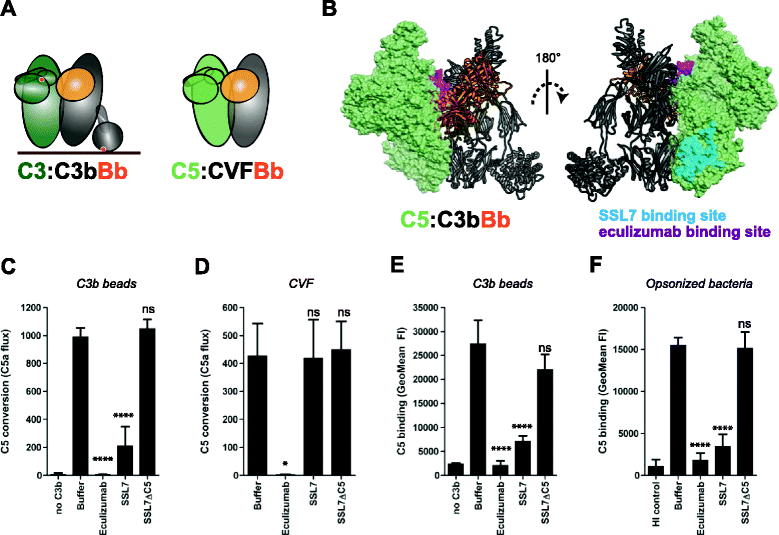
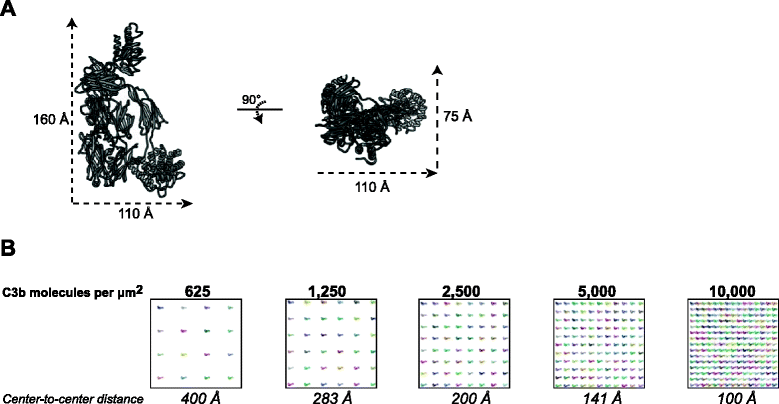
Results: Alternative pathway (AP) C5 convertases were generated on small streptavidin beads that were coated with purified C3b molecules. Site-specific biotinylation of C3b via the thioester allowed binding of C3b in the natural orientation on the surface. In the presence of factor B and factor D, these C3b beads could effectively convert C5. Conversion rates of surface-bound C3b were more than 100-fold higher than fluid-phase C3b, confirming the requirement of a surface. We determine that high surface densities of C3b, and its attachment via the thioester, are essential for C5 convertase formation. Combining our results with molecular modeling explains how high C3b densities may facilitate intermolecular interactions that only occur on target surfaces. Finally, we define two interfaces on C5 important for its recognition by surface-bound C5 convertases.
Conclusions: We establish a highly purified model that mimics the natural arrangement of C5 convertases on a surface. The developed model and molecular insights are essential to understand the molecular basis of deregulated complement activity in human disease and will facilitate future design of therapeutic interventions against these critical enzymes in inflammation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

